2025 AIChE Annual Meeting
(536g) Multimodal in Situ Characterization Reveals Pd Surface Stability through Structural Dynamics in Acidic Media for Oxygen Electroreduction
Authors
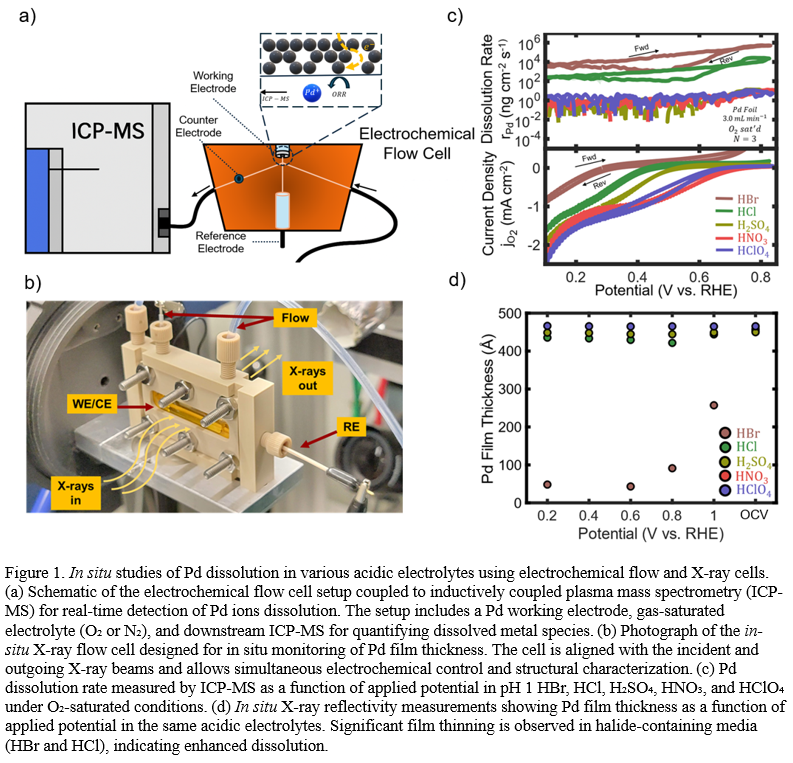
This study probes the electrochemical behavior of Pd metal in various electrolytes, focusing on anion effects on ORR in acidic media. For Pd surfaces, ORR activity decreases in the presence of oxyacid and halide anions in the following order: HClO4 > H2SO4> HNO3 > HCl > HBr. On-line inductively coupled plasma mass spectrometry (ICP-MS) reveals variations in Pd stability during ORR, closely mirroring the activity trends (HClO4 > HNO3 > H2SO4 > HCl >> HBr). In halide electrolytes, the Pd dissolution rate at 0.8 VRHE is approximately 106 ng cm-2s-1, whereas, in oxyacidic electrolytes, it is significantly lower at ~1 ng cm-2s-1. Ex situ atomic force microscopy (AFM) demonstrates anion-dependent surface roughening, specifically in Br- electrolytes where signs of Pd dissolution are apparent. In contrast, for the oxyacid anions, post-ORR surface roughness remains minimal (roughness factor of about 1.02) indicating a low probability of anion interactions with the surface. Ex situ X-ray photoelectron spectroscopy confirms the assumed minimal shifts in oxidation states of the Pd 3d orbital in high-resolution scans, suggesting that the Pd surface undergoes alterations under operating conditions.
Synchrotron-based X-ray techniques, including X-ray Reflectivity (XRR) and grazing incidence X-ray diffraction (GIXRD), are employed to probe the in situ electrocatalyst surface during ORR. In oxyacidic media, the electrodes do not exhibit significant roughening, whereas halide electrolytes induce transient film loss, observed via Kiessig fringe changes and corroborated by ex situ AFM measurements. The extent of Pd film thickness loss with decreasing applied potential follows the trend: HClO4 ~ HNO3 < H2SO4 < HCl << HBr, which closely aligns with the stability trends observed in on-line ICP-MS.
Furthermore, GIXRD measurements reveal anion-dependent lattice strain in polycrystalline Pd electrocatalysts, where H₂SO₄ electrolytes induce greater tensile strain compared to less competitively adsorbing oxyacidic media, highlighting anion adsorption-dependent lattice strain during ORR. Additionally, certain halide electrolytes promote the formation of high Miller index facets compared to oxyacidic media, demonstrating significant surface reconstruction during potential sweeps and further confirming anion-dependent restructuring during ORR.
By extensively studying anion effects of Pd electrocatalysts, we aim to advance the fundamental understanding of ORR electrocatalyst reconstruction and stability trends under acidic operating conditions. State-of-the-art proton exchange membranes in PEMFCs contain an anionic functional group that influences local interfacial environment. Studying Pd dissolution and surface dynamics as a function of anion microenvironment can help inform strategies to tailor ionomer functional group identity, helping to mitigate competitive adsorption and electrocatalyst degradation-ultimately contributing to the design of efficient and stable PEMFCs for a sustainable future.