2025 AIChE Annual Meeting
(254a) Multi-Scale Simulation Guided NH3 Decomposition over Carbide and Nitride Systems
Authors
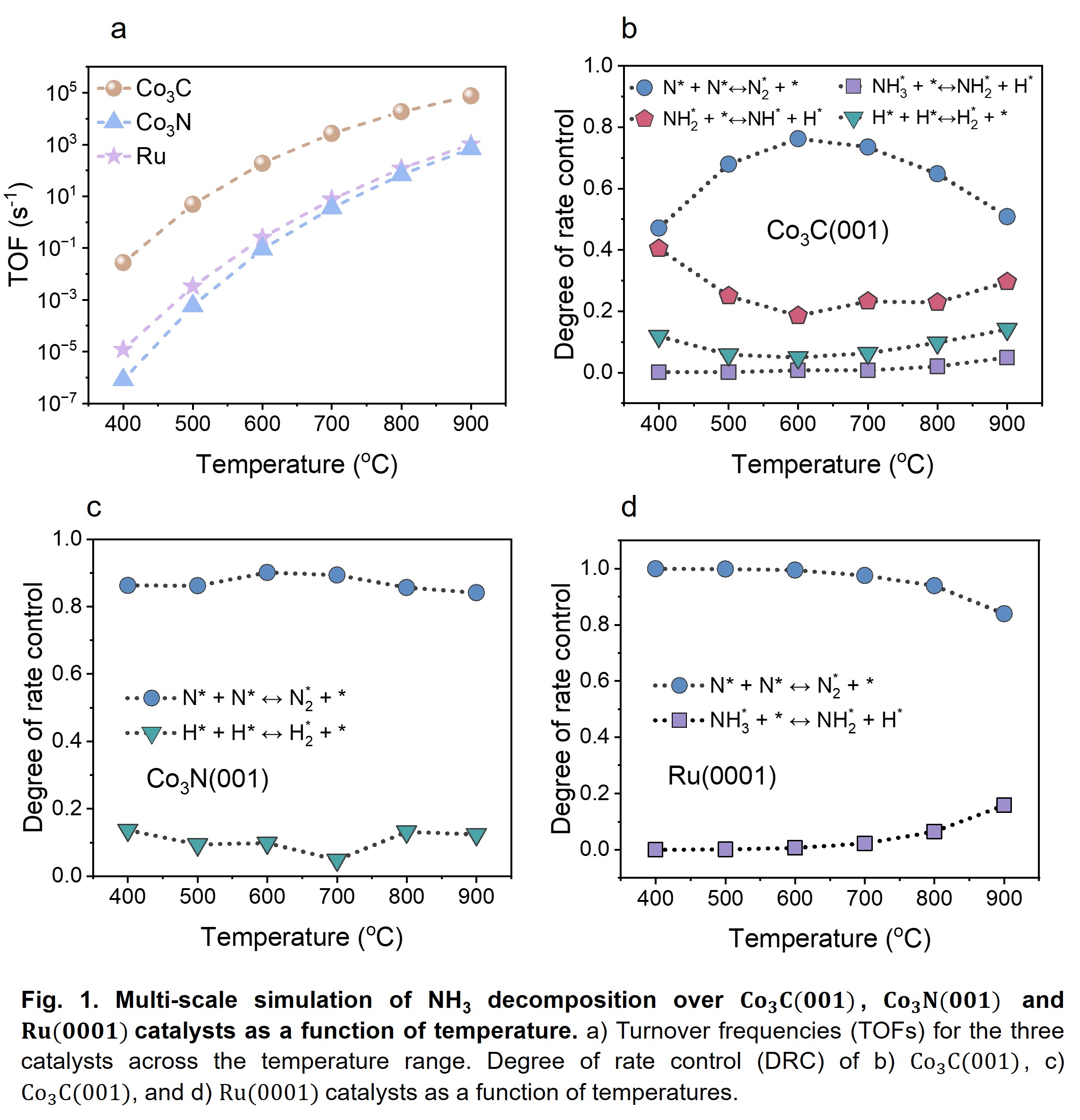
Our results reveal that the (001) facets of Co3C and Co3N are the most stable, with C-terminated Co3C(001) and N-terminated Co3N(001) surfaces being more favorable than their Co-terminated counterparts. Notably, subsurface vacancies form more readily than surface vacancies, facilitated by lattice relaxation and additional surface layers, particularly under hydrogen-rich conditions. We then established microkinetic modeling (MKM). Our MKM results indicate that Co3C(001) exhibits (Fig. 1a) times higher turnover frequency (TOF) than Co3N(001) and times higher TOF than Ru(0001), highlighting its superior catalytic performance. Unlike Ru(0001), where N≡N coupling is the sole rate-determining step (RDS), Co3C(001) follows a distinct mechanism where N≡N and H–H couplings, along with NH2 dehydrogenation, contribute as RDS. For Co3N(001), N≡N and H–H couplings dominate the RDS (Figs. 1b-d). These findings reinforce the potential of transition metal carbides and nitrides as alternative catalysts for NH3 decomposition, benefiting from their dual binding sites, high electronic conductivity, and structural versatility.
References
- Li, H., et al., ACS catalysis, 2020, 10, 11814-11821.
- Tian, D. et al., Chemical Society Reviews, 2021, 50, 12338-12376.
- Hansgen, D. A. et al., Nature Chemistry, 2010, 2, 484-489.