2025 AIChE Annual Meeting
(522e) Modulating Surface Charge to Control Adsorbate Binding Beyond Periodic Trends
Authors
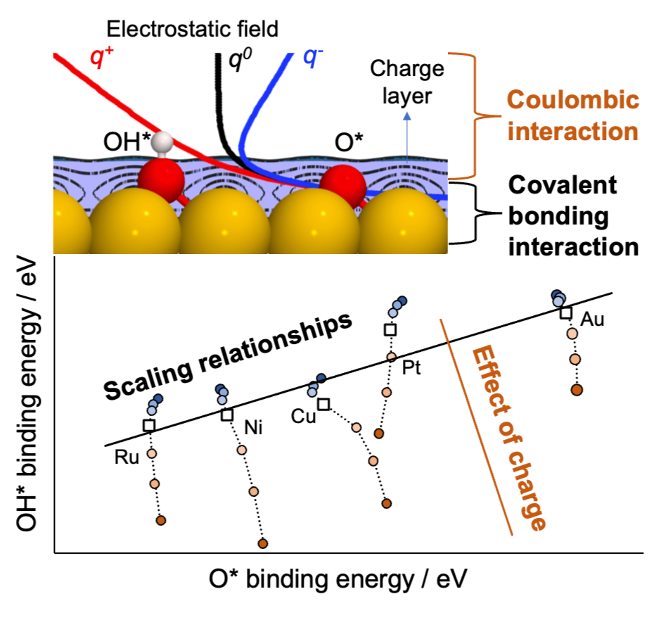
In this study, we show that binding energy changes up to ca. 2.0 eV can be achieved by applying net positive charges of +0.17 h+ to transition metal surfaces (e.g., Ru, Pt). Conversely, only small changes in BE are observed for net negative charges across various d-block elements (e.g., Pt, Ru, Cu, Ni, Au). Atomic adsorbates like oxygen exhibit minimal BE changes in response to applied charges, suggesting the role of adsorption configuration in charge sensitivity. For instance, atomic oxygen remains within a charge band atop the metal layer, unaffected by the external electric field. In contrast, vertically aligned adsorbates (e.g., COOH, CH3O) show significant BE changes due to interactions between their dipole moments and charged surfaces.
We also investigated the validity of LSRs for charged surfaces. Our analysis reveals that LSRs are valid for metal surfaces under charge, contingent on the choice of descriptor. Using the BE of atomic adsorbates (e.g., oxygen, carbon) to predict the BE of oxygenates and CHx species fails to yield expected correlations. Improved correlations are achieved using the BE of OH and CH as descriptors for oxygenated and CHx species, respectively, over the same metal surfaces.