2025 AIChE Annual Meeting
(665h) Modular Control of a Continuous Synthesis and Purification Line
Authors
From research and industry there are multiple examples for active process control of single unit operations and integrated pharmaceutical manufacturing lines (Destro and Barolo[1] , Sacher et al.[2]). However, these studies mainly focus on solid dosage manufacturing. Commonly, the critical process parameters (CPPs) of each unit operation are directly assigned by one specific rather complex plant-wide controller. This approach implies two major setbacks. First, one plant-wide model requires extensive testing or simulation. Second, the structure of the controller is fixed and cannot be adapted to any changes in the plant setup without significant effort.
Within this talk, we present a modular control concept of an integrated pharmaceutical manufacturing line, which allows high flexibility in terms of process configuration and control objectives. The work is focused on the upstream and purification section and includes a continuous synthesis based on the Simmons-Smith reaction, a solid-liquid separation by means of a decanter centrifuge and a liquid-liquid-extraction by means of a stirred counter-current extraction column.
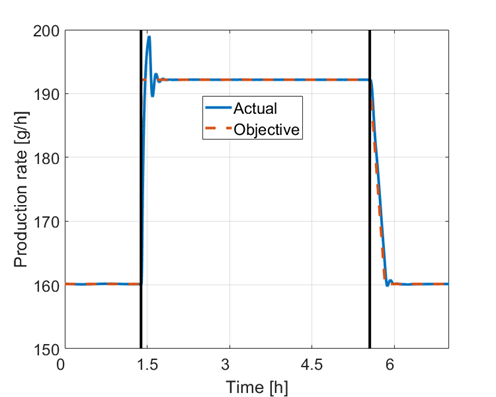
The talk will present the modeling and validation for each unit operation. The control structure is capable of achieving overall plant control targets while keeping flexibility on the unit operation level. It is designed as a multi-level modular control to allow improvements of each unit operation and its own controller. The unit operation control targets for each unit operation are derived from the overall control targets. Each unit operation controller calculates the actual CPPs based on the unit operation control targets. The unit operation controllers will be briefly introduced, covering options from feed-forward to model predictive controllers (MPC). The capability of the presented control concept will be demonstrated in simulation, where the validated non-linear process models serve as the plant. Figure 1 shows the production rate for a use case with two changes of the desired production rate. The actual production rate is following the overall control target in both cases.
The demonstrated structure simplifies implementation at industry plants due to its modular approach, which allows adaptations of single unit operations or changes in equipment, without the need for reparameterization or even re-development of a tailored control concept for one specific plant. As a result, the cost in the case of adaptations is significantly lower compared to existing demonstrations. Further, the study successfully demonstrates the control of a synthesis-purification line, which includes resource intensive unit operations and therefore leads to high potential in the plant wide resource efficiency.
[1] Destro, F., Barolo, M., 2022. A review on the modernization of pharmaceutical development and manufacturing - Trends, perspectives, and the role of mathematical modeling. International journal of pharmaceutics 620, 121715. 10.1016/j.ijpharm.2022.121715.
[2] Sacher, S., Poms, J., Rehrl, J., Khinast, J.G., 2022. PAT implementation for advanced process control in solid dosage manufacturing - A practical guide. International journal of pharmaceutics 613, 121408. 10.1016/j.ijpharm.2021.121408.
Figure 1: Production rate of the integrated upstream pharmaceutical production plant over time.