2025 AIChE Annual Meeting
(321a) Modifying Ag-Electrode Catalyst Selectivity of Picloram Reduction Using Surface Steps
Authors
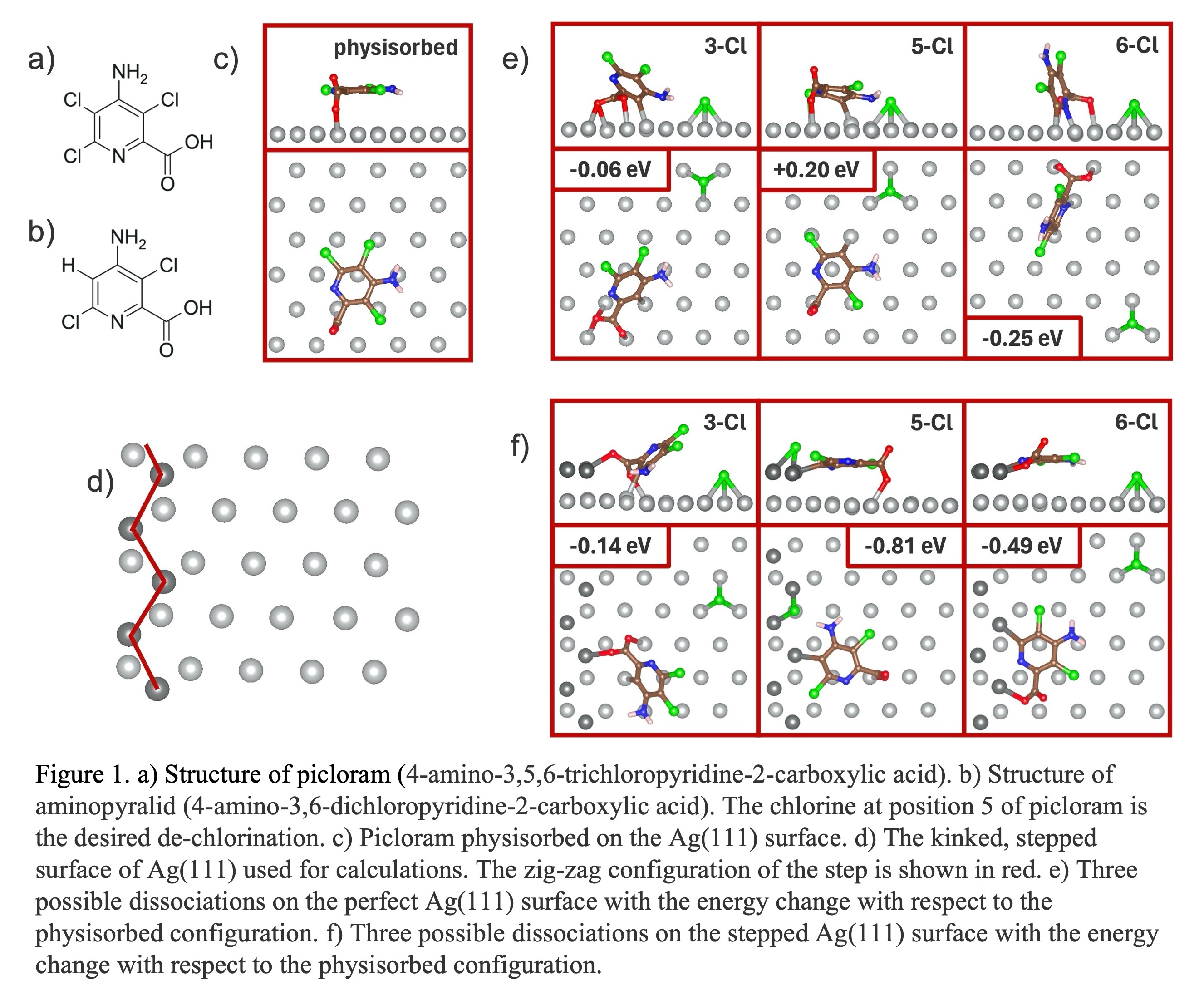
Density functional theory was used to determine the standard reduction potential of the first outer-sphere electron transfer in implicit water solvent. The experimental solution pH was much larger than the pKa of picloram so the deprotonated anion was used. The calculated reduction voltages were similar for any chorine dissociation, suggesting the necessity of a catalyst.
Slab models were constructed to compare chlorine dissociation on flat and stepped Ag(111) surfaces. On the perfect surface, the most stable physisorption configuration was with the aromatic ring parallel to the surface. The bond dissociation energy on the flat surface and by the surface step was then compared. On the perfect surface, this energy cost was +0.20 eV for 5-chlorine and was -0.25 eV for 6-chlorine. 6-chlorine dissociation was preferred, and 5-chlorine dissociation was not spontaneous. Contrastingly, the zig-zag configuration of Ag atoms on a kinked surface step allows bonding with the radical intermediate while avoiding possible steric effects from the large chlorine and amine groups. The bond-breaking energy change for 5-chlorine was -0.81 eV, and for 6-chlorine was -0.49 eV.
Atomic steps are inherent to nanoparticle catalysts due to the high curvature, and density of surface steps will decrease with increasing particle size. Roughening methods, like anodic pulses, can increase step density and defects to modify reaction selectivity. Ultimately, this knowledge of picloram reduction selectivity on kinked stepped surfaces can illuminate experimental electrocatalyst design to produce the safer herbicide aminopyralid.