2025 AIChE Annual Meeting
(638b) Modeling of the Kinetics of Tandem Chain Scission/Aromatization
Authors
Jiankai Ge - Presenter, University of Illinois at Urbana-Champaign
Jiakai Sun, University of California Santa Barbara
Danielle Burns, University of California Santa Barbara
Yu-Hsuan Lee, University of California Santa Barbara
Mahdi Abu-Omar, University of California, Santa Barbara
Andreas Heyden, University of South Carolina
Baron Peters, University of Iliinois
Susannah Scott, University of California, Santa Barbara
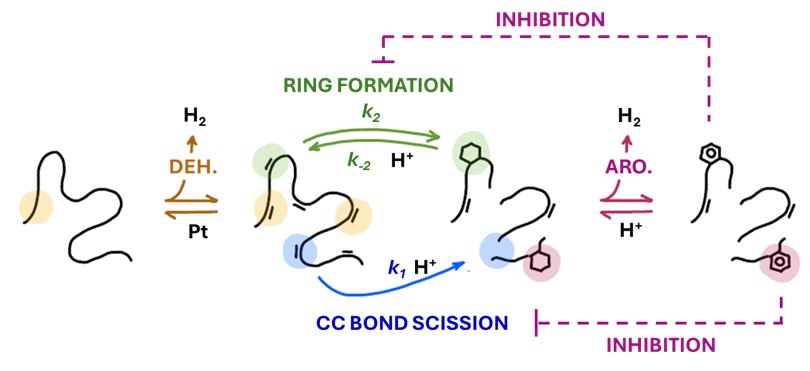
The catalytic depolymerization of polyethylene (PE) to alkylaromatics using bifunctional catalysts such as Pt/SiO2 -Al2 O3 offers a promising pathway for sustainable plastic upcycling. The tandem reactions of C-C bond scission and aromatization occur without external hydrogen under mild conditions by redistributing PE-derived hydrogen. However, external hydrogen pressures (PH2 ) significantly influence both the reaction kinetics and product selectivity. At moderate pressures, PH2 enhances the depolymerization rate, increases alkylbenzene yield, and suppresses polyaromatic formation.
In this study, a kinetic model incorporating the quasi-equilibria of dehydrogenation and aromatization was developed to describe the experimental observations. The model accounted for the competitive adsorption of aromatic products on active catalytic sites, elucidating a pseudo-zeroth-order dependence of aromatic yields and the non-monotonic dependence of C-C bond scission rates on PH2 . Fitting the model to data yields rate constants and equilibrium constants from the experimental results. The insights provide a mechanistic understanding of the role of PH2 in enhancing depolymerization rates while optimizing aromatics yield.