2025 AIChE Annual Meeting
(591d) Model Construction of Water Partition for Continuous Esterification Process Design
Authors
Fatty acid methyl ester (FAME) is widely used as a renewable biodiesel alternative to diesel fuel, and global production of FAME is increasing at a compound annual growth rate (CAGR) of 5.7%¹. Currently, FAME is produced primarily through transesterification of triglyceride, the main component of cooking oil and waste cooking oil. However, the use of cooking oil is problematic due to competition with food supply, and waste cooking oil has recently been increasingly diverted to the production of sustainable aviation fuel (SAF). Therefore, we proposed an alternative FAME production process using fatty acid (FA), which is a waste by-product generated in large quantities during edible oil refining, as shown in Eq.(1). Many researchers have studied FAME production by esterification of FA using various catalysts2-5. However, as shown in Table 1, the limitation due to the reverse reaction, which requires the addition of excess MeOH or the removal of water to achieve high conversion, has been a major challenge for practical application. We have discovered that by using a solid acid catalyst, a porous type cation-exchange resin, the complete conversion can be achieved without both the addition of excess MeOH and the removal of water. Furthermore, we found that the partitioning of water between the resin and bulk phases varies with the MeOH/FA molar ratio of the feed solution7. This finding suggests the possibility of developing a continuous esterification process that does not require catalyst regeneration by controlling the composition of the feed solution to prevent water accumulation within the resin catalyst. The aim of this study is to quantitatively elucidate the relationship between feed composition (kinds of FA and MeOH/FA molar ratio) and the water partitioning behavior, and to construct a mathematical model that can predicts the water partitioning behavior. First, batch esterification was performed using various FAs, and water partitioning after esterification was measured. Next, a competitive partition equilibrium model was constructed to clarify the effect of FA type on water partitioning.
2. Experimental
Diaion PK208LH (a porous strong acid cation-exchange resin), reported its high esterification activity, was used as the catalyst after swelling in MeOH. Seven FAs, decanoic acid, myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, which are commonly found in vegetable oils, were used alone. To keep the amount of water produced and the amount of resin constant, the MeOH/FA molar ratio in the feed solution was adjusted from 1 to 10 by varying the amount of MeOH. In each experiment, the esterification was allowed to proceed completely by stirring well at 50°C. The water content in the bulk solution after completion of the reaction was then measured with a Karl-Fischer moisture meter. The concentrations of each component in both the bulk and resin phases were analyzed by gas chromatography (GC).
3. Construction of a competitive partition equilibrium model
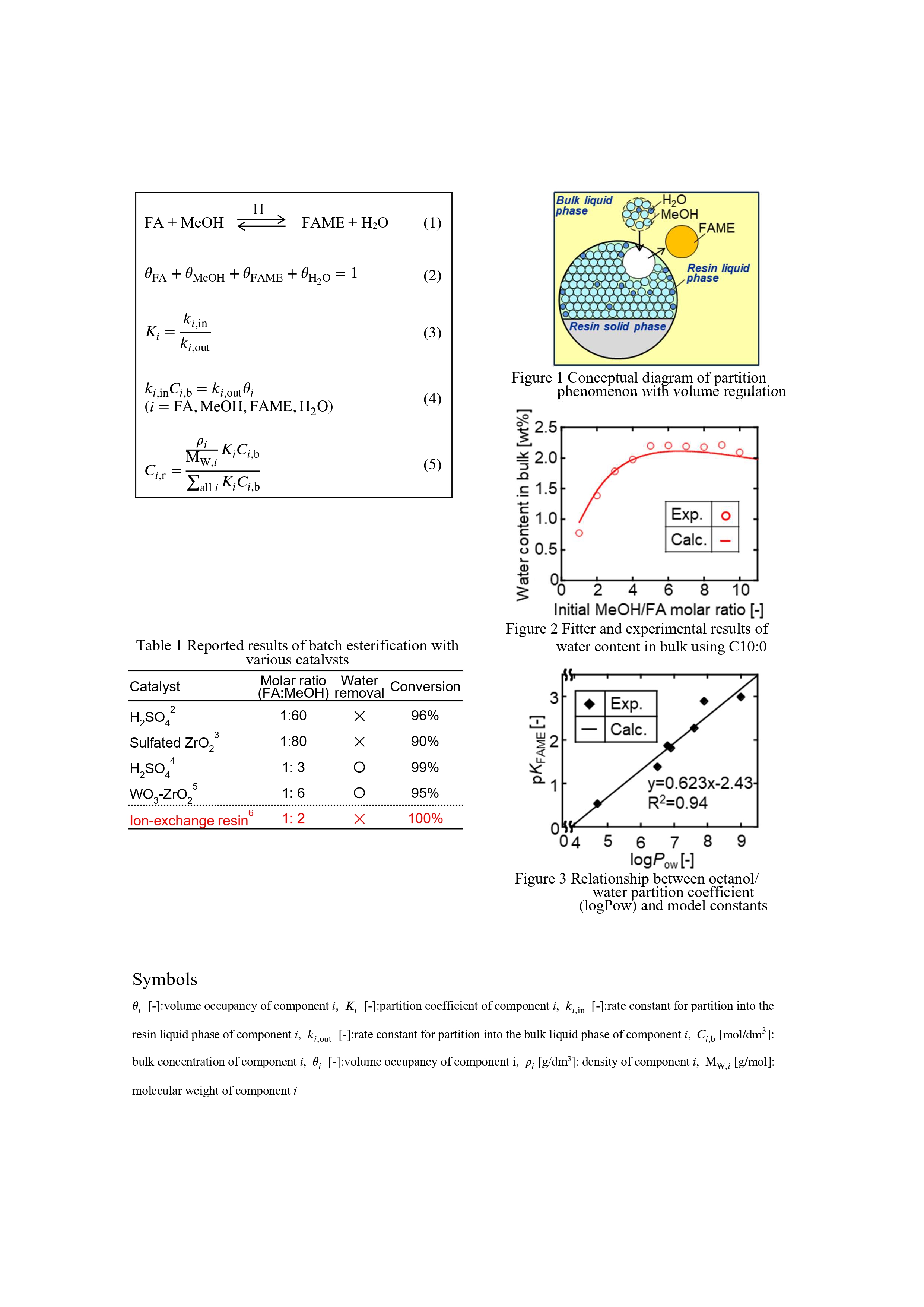
Based on the experimental results, the following assumptions were made in considering the partition behavior of each component in the system without solvent. (i) Three phases are considered: bulk liquid phase, resin liquid phase, and resin solid phase, and each component exists in the bulk liquid phase and the resin liquid phase. (ii) The volume of the resin liquid phase is constant and all its space is occupied by reactants and products. A conceptual diagram of the competitive partition equilibrium model is shown in Fig.1. The resin liquid phase is constant, a constraint on the total volume of absorbable components was introduced using volume occupancy of components (Eq.(2)). The partition coefficient for each component was defined as the ratio of the uptake rate constant into the resin liquid phase to the discharge rate constant into the bulk liquid phase (Eq.(3)).
GC analysis showed that after sufficient reaction, the FA concentration was below the detection limit. Therefore, it is assumed that the esterification proceeds completely, all FA is converted to FAME and water, and excess MeOH remains. Since the system was reached equilibrium, a partition equilibrium between the resin liquid phase and the bulk liquid phase was assumed for each component (Eq.(4)). The Langmuir equation describing the relationship between the resin and bulk concentrations for each component (Eq.(5)) was derived. The unknown constants of the model were the partition coefficients of the three components: MeOH, FAME, and H₂O. These were estimated by fitting with the experimental results of the water content in bulk solution.
4. Application of partition equilibrium model
Figure 3 shows the results of applying the model using capric acid (FA with a carbon chain length of 10 and zero double bonds) as an example. The abscissa represents the MeOH/FA molar ratio in the esterification experiment, and the ordinate is the equilibrium water content in bulk liquid phase after the complete reaction. The fitted results (lines) well represent the experimental results (symbols), and this trend was observed for all fatty acids.
Next, we examined the relationship between the molecular polarity and the estimated partition coefficients of FAME obtained using various FAs. Figure 4 plots the estimated values of each partition coefficient against the octanol/water partition coefficient8, an indicator of molecular polarity. A strong correlation was observed, suggesting the possibility of predicting water partition behavior from the polarity of FAME. Therefore, this model can provide valuable insights for designing the continuous esterification process of waste fatty acids, which are actually fatty acid mixtures.
5. References
[1] Transparency Market Research, Inc. < https://www.transparencymarketresearch.com/> (Accessed April 7,2025)
[2]M.Berrios et al.,Fuel,86,2383(2007)
[3]X.Chen et al.,J.Phys.Chem.C.,111, 18731(2007)
[4]L.L. Izabelly et al.,Ind.Eng.Chem.Res.,47,6885(2008)
[5]P.Mongkolbovornkij et al.,Fuel Proc.Tech.,91,1510(2010)
[6]N.Shibasaki-Kitakawa et al.,Fuel,139,11(2015)
[7]K.Hiromori et al., AOCS 2024 Annual Meeting & Expo (2024)
[8]Pub Chem <https://pubchem.ncbi.nlm.nih.gov/> (Accessed April 3,2025)