2025 AIChE Annual Meeting
(562d) Microwave-Driven Non-Oxidative Catalytic Conversion of Methane to Ethylene
Authors
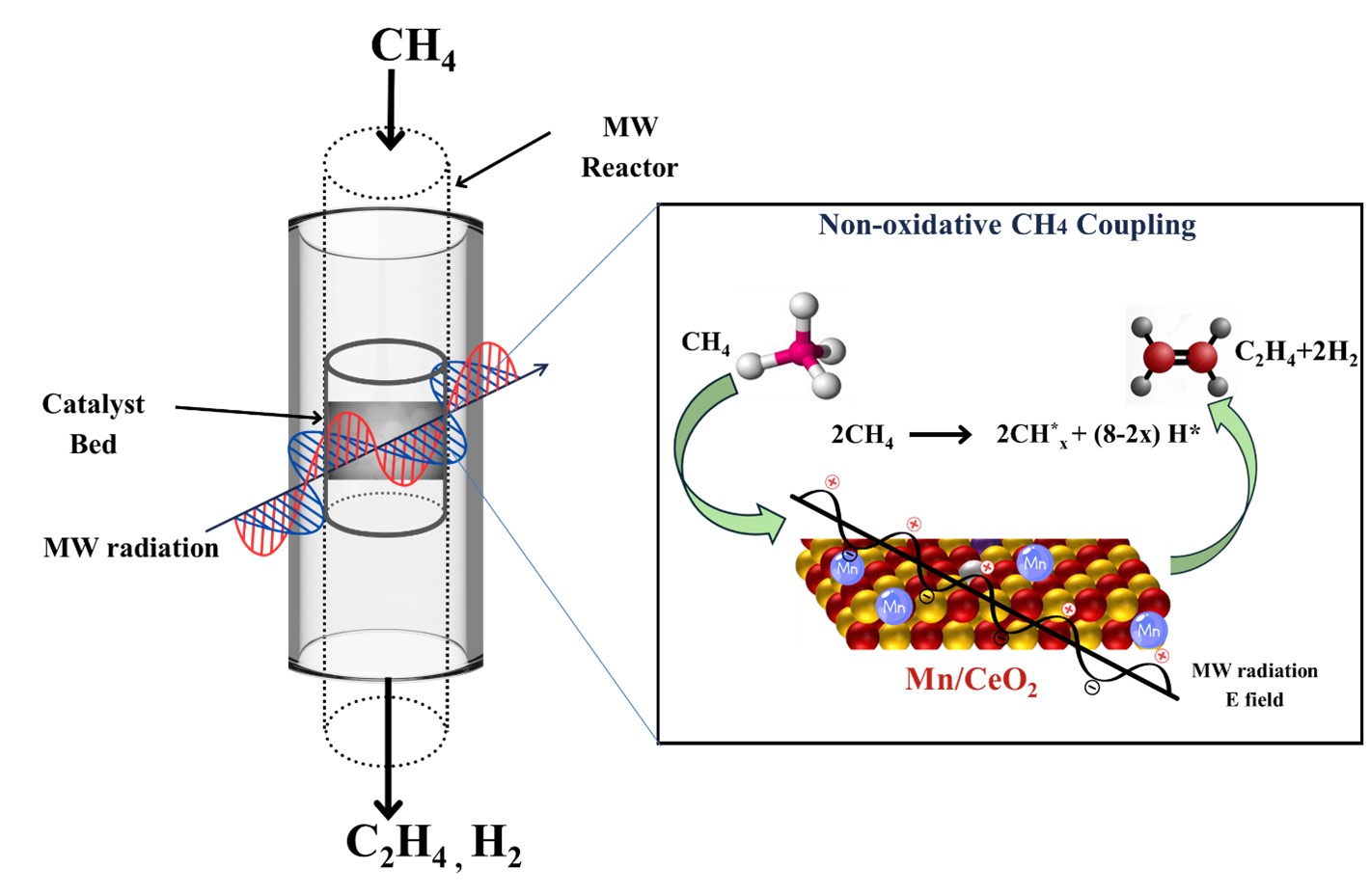
Ethylene is one of the vital chemicals used in a variety of applications in the industries as solvent, fertilizers, refrigeration systems, and chemical feedstocks etc. The commercial production of ethylene via steam cracking is an extremely energy-intensive process and is heavily dependent on fossil fuels. Recent advancements in microwave-assisted catalytic synthesis of ethylene offer a promising, energy- efficient and eco-friendly alternative to conventional methods. Microwave irradiation selectively heats the catalyst surface which significantly reduces temperature, pressure and reaction time. This work investigates the direct, non-oxidative conversion of methane to ethylene over manganese (Mn) and molybdenum (Mo)-based catalysts at different reaction temperatures, using cerium oxide (CeO2) as a support material. CeO2 was selected due to its excellent microwave susceptibility and its capacity to facilitate the formation of metal-Ce-O bonds, promoting the high dispersion of metal particles during microwave-assisted chemical synthesis. Additionally, the effect of incorporating cesium (Cs) as a promoter on ethylene selectivity and methane conversion were explored. In the presence of a promoter, 90% selectivity towards C2s was achieved, with methane conversion exceeding 30%. The results suggest that the incorporation of Cs can further enhance both ethylene selectivity and methane conversion, thereby improving the overall catalytic performance.
Keywords: Ethylene, Microwave-assisted catalytic synthesis, non-oxidative, Methane, selectivity
Figure: Schematic of Microwave-driven Non-Oxidative Catalytic Synthesis of Ethylene from Methane