2025 AIChE Annual Meeting
(154f) Microwave Based Fluidized Bed Reactor
Authors
Microwave heating is based on the ability of molecules with a dipole moment to absorb microwave energy and effectively convert it into heat. Microwave heating occurs via direct molecular interactions with the applied electromagnetic radiation, therefore it provides advantages of instantaneous and volumetric heating without the heat transfer restrictions and heat losses associated with the conventional conductive or convective heating modes [1]. Microwave heating offers a number of advantages such as volumetric heating, rapid heating, non-contact heating, quick start-up and stopping, low cost and portability of equipment and processes [2].
This talk gives an overview how microwave heating impacts heat transfer in fluidized bed reactors. Microwaves directly heat the particles in the bed, resulting in more efficient heat transfer compared to conventional heating methods that rely on external sources. The penetration of microwaves allows for more uniform heating throughout the reactor which can prevent the hot spot formations leading to a more consistent temperature distribution across. The rapid heating of particles can improve the overall heat transfer rates between the gas and solid phases. As particles heat up directly, they can effectively transfer heat to the surrounding gas. Better uniformity in heating, can lead to improved reaction kinetics in processes.
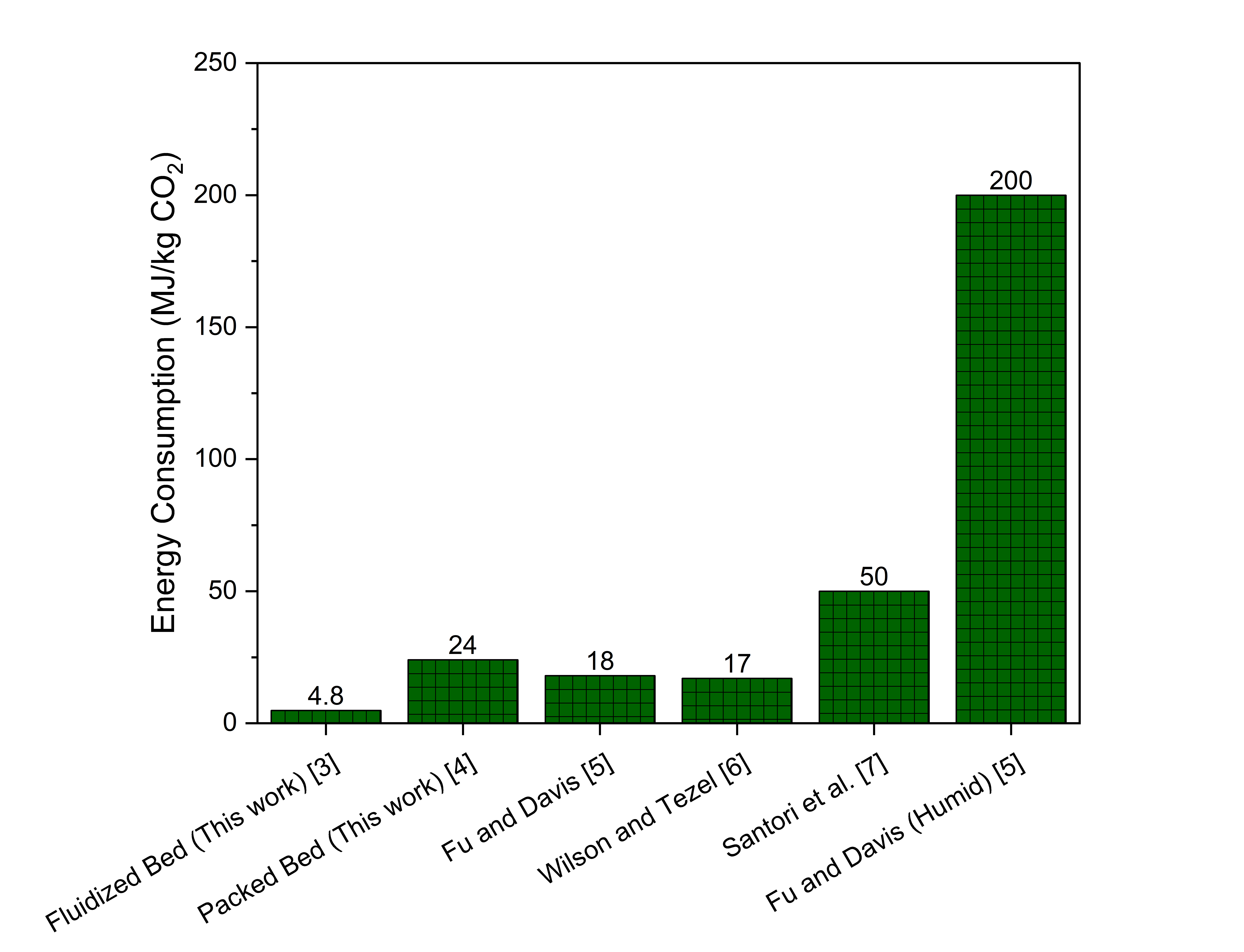
Results from regeneration of solid sorbent, Zeolite 13X, for CO2 removal from air is shown in this work using a mono-mode microwave system in fluidized bed reactor configurations [3, 4]. The results are compared to packed bed under the same microwave conditions. The effects of varying initial microwave power (4 – 30 W) were investigated with target regeneration temperatures range of 33 – 100 °C on CO2 adsorption and desorption behavior, with a focus on sorption capacity and energy efficiency. Fluidization enhanced heat distribution within the sorbent by promoting uniform thermal contact, while the volumetric nature of microwave heating led to effective CO2 release. Results showed that lower regeneration temperatures significantly reduced energy consumption, emerging as the most influential variable. Furthermore, the fluidized bed enabled higher airflow rates, reducing the time required for complete desorption. The temperature uniformity in both packed bed and fluidized bed reactor configurations are investigated with an infrared thermal camera and a pyrometer. The findings confirm that efficient CO2 desorption is achievable under low-temperature, low-power conditions when fluidization is applied. As compared in Figure 1, energy consumption of DAC processes have been investigated and they were found in a range between 17 and 200 MJ/kg CO2 [4-7]. In this work, the energy consumption for regeneration process were found to be 4.8 and 24 MJ/kg CO2 under fluidized and packed bed conditions, respectively.
Figure 1: Energy requirement comparison for regeneration process using Zeolite 13X for DAC applications
Overall, the interaction between microwave heating and multiphase flows in fluidized beds can enhance processing efficiency, but it requires optimization. While microwave based fluidization provides an effective way of overcoming the non-uniform heating problems in conventional pure microwave heating, future works will investigate the effect of scale up in this system.
References
- Bougie, F. and X. Fan, Microwave regeneration of monoethanolamine aqueous solutions used for CO2 capture.International Journal of Greenhouse Gas Control, 2018. 79: p. 165-172.
- Sun, J., W. Wang, and Q. Yue, Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials, 2016. 9(4): p. 231.
- Erguvan, M., et al., An experimental study on microwave-assisted direct air capture of CO 2 under fluidized bed conditions. Sustainable Energy & Fuels, 2025.
- Boylu, R., M. Erguvan, and S. Amini, Investigation of microwave-based CO2 regeneration in a packed bed reactor for Direct Air Capture. Chemical Engineering Research and Design, 2024. 212: p. 391-404.
- Fu, D. and M.E. Davis, Toward the feasible direct air capture of carbon dioxide with molecular sieves by water management. Cell Reports Physical Science, 2023. 4(5).
- Wilson, S.M. and F.H. Tezel, Direct dry air capture of CO2 using VTSA with faujasite zeolites. Industrial & Engineering Chemistry Research, 2020. 59(18): p. 8783-8794.
- Santori, G., et al., Adsorption artificial tree for atmospheric carbon dioxide capture, purification and compression.Energy, 2018. 162: p. 1158-1168.