2025 AIChE Annual Meeting
(226a) Microwave Assisted Catalytic Conversion of Bioethanol to Ethylene Using Mn-Zn/HZSM-5
Authors
Changle Jiang, West Virginia University
Brandon Robinson, West Virginia University
Jianli Hu, West Virginia University
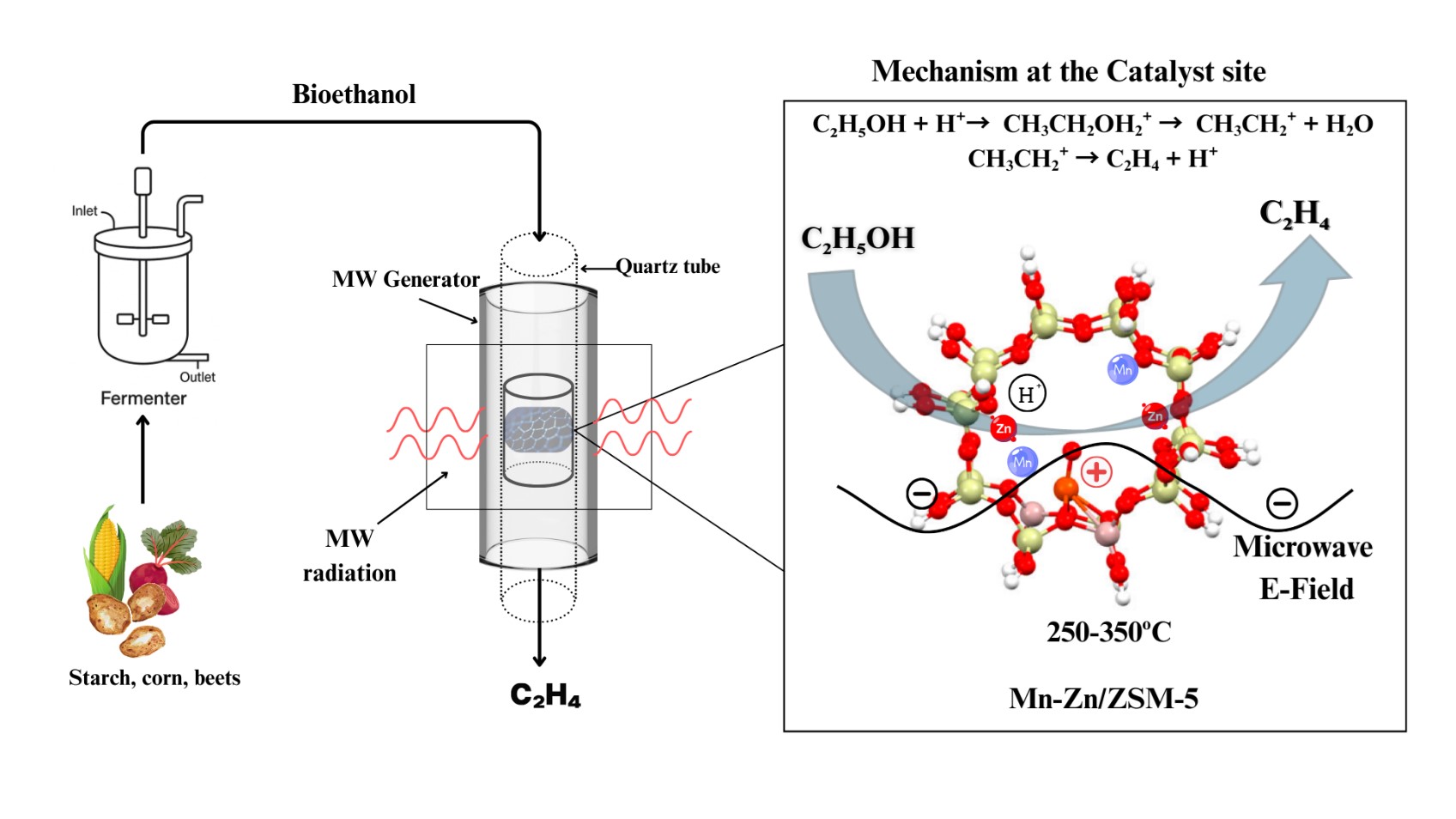
Ethylene is one of the most important chemicals used in the chemical, textile, and automotive industries. Steam cracking of naphtha and separation of refinery gases are used in the industrial production of ethylene. The sustainable bioethanol that derives from renewable biomass materials, including sugarcane, corn, and lignocellulosic residues, enables catalytic dehydration to produce ethylene. The research analyses the ethanol-to-ethylene (EtE) process implementation through a reactor system that heats the catalyst bed using dielectric effects instead of traditional heat transfer methods. This study investigates the ethanol-to-ethylene (EtE) process using a microwave-assisted fixed-bed reactor, which selectively heats the catalyst bed via dielectric interactions rather than relying on conventional heat transfer. In this study, two types of zeolites with different Si/Al ratios (CBV 400 and CBV 3024E) were modified with metal combinations of manganese and zinc, with a total metal loading of 10 wt%. The role of acid site density, support structure, and metal synergy was systematically studied to optimize ethylene selectivity and minimize byproducts such as diethyl ether and acetaldehyde. Particular attention was given to the influence of Brønsted and Lewis acid sites, introduced and modulated by metal incorporation, in steering the reaction pathway and stabilizing key intermediates. The reactions in this study were carried out at temperatures of 250, 300, and 350 °C. The catalyst containing 8 wt% Mn and 2 wt% Zn exhibited the best performance across both supports, achieving ethanol conversions of 95% and ethylene selectivity as high as 99.5%, while maintaining excellent thermal and catalytic stability. These findings demonstrate the potential of rationally tuned acid sites and microwave energy coupling to enable efficient, low-temperature ethylene production from renewable feedstocks.
Keywords: Ethylene, Bioethanol Dehydration, HZSM-5, Microwave Reactor, Catalyst Acidity, Mn-Zn Synergy
Figure: Microwave-Assisted Ethanol Dehydration over Mn-Zn Modified HZSM-5 Showing Reaction Pathway at Catalyst Site