2025 AIChE Annual Meeting
(668d) Microwave-Assisted Regeneration of Purolite and Lewatit Adsorbents for Direct Air Capture
Authors
To eliminate any volatiles (CO2, H2O, etc.) from the sorbent, 3 g of the sample was placed in a vacuum oven for at least 12 hours at 120 °C. A borosilicate glass reactor with inner and outer diameters of 16 and 18 mm, respectively, was used for all experiments. During the regeneration process, a compact 200 W microwave unit, consisting of a solid-state microwave generator and a cavity, was employed. The microwave unit could adjust the frequency between 2.4-2.5 GHz. The sample's temperature was controlled using a pyrometer, which was also connected to the microwave generator unit to not only read the temperature but also adjust the forward power based on the regeneration temperature. Twenty-six DAC experiments were conducted in three stages (pre-treatment, adsorption, and desorption) to analyze the effect of temperature and microwave power on both amine sorbents. In the adsorption process, the feed gas was supplied at four different relative humidity (dry to 75%, with 25% increments). During the regeneration process, the microwave power was varied at two different levels (15 and 30 W), and the regeneration temperature was tested under five different conditions (40-120 °C, with 20 °C increments) for each microwave power.
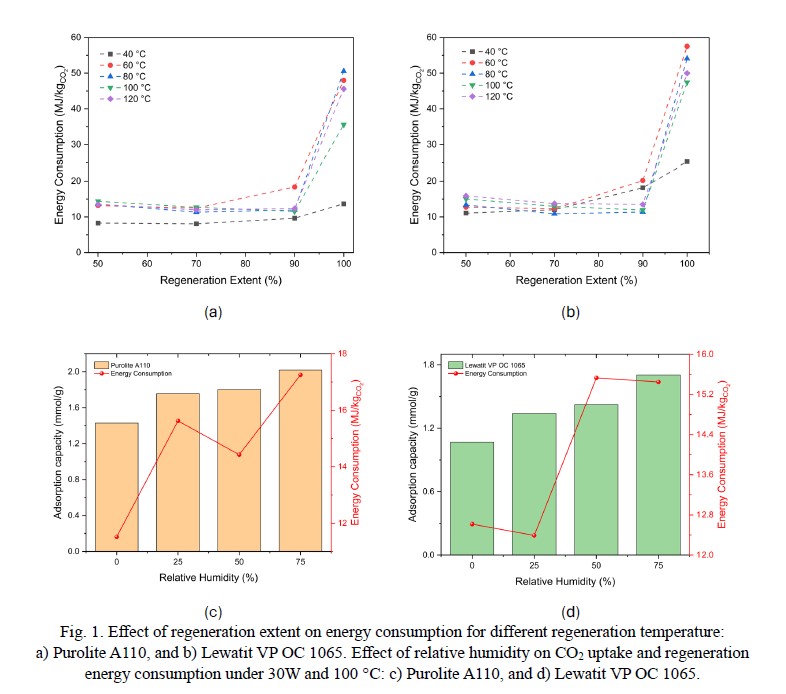
The findings demonstrate that microwave regeneration is a viable method for amine-solid sorbents, achieving microwave absorption energy efficiency between 33% and 44%, with a regeneration recovery rate exceeding 83% for both sorbents. In terms of performance, Purolite A110 surpasses Lewatit® VP OC 1065, offering 26% greater adsorption capacity under dry conditions (1.43 mmol/g vs. 1.07 mmol/g), 20% higher CO2 productivity, and 30% reduced microwave energy usage. Additionally, a sensitivity analysis was conducted to explore the critical performance indicators of the processes as shown in Fig. 1a and Fig. 1b. Implementing partial regeneration (90% desorbed) leads to microwave energy savings of 66-79%, with minimal impact on recovery efficiency and working capacity. Moreover, Fig. 1c and Fig. 1d illustrates the effect of relative humidity on CO2 adsorption capacity and regeneration energy consumption for Purolite A110 and Lewatit VP OC 1065 under 30 W and 100 °C. As expected, the CO2 uptake increases with rising humidity levels, up to 51% for Purolite A110 and 59% for Lewatit VP OC 1065. However, this enhanced adsorption capacity comes at an energy cost, with the regeneration energy penalty rising by 49.8% and 59.5%, respectively. This highlights the sensitivity analysis between the CO2 uptake and the energy required for regeneration seeking for energy-efficient CO2 capture technology.
The results indicate a strong interaction between microwave irradiation and the amine-solid sorbents, concluding both are MW-absorbed materials, making the regeneration process feasible. The CO2 capacities for Purolite A110 and Lewatit VP OC 1065 were found of 1.44 mmol/g and 1.07 mmol/g under dry condition, respectively. Although, the CO2 uptake can reach up to 2.02 mmol/g for Purolite A110 and 1.7 mmol/g for Lewatit VP OC 1065 by increasing the relative humidity to 75%, however, with a penalty in energy consumption. Finally, this work demonstrates the feasibility of using microwaves in the regeneration of amine solid sorbents for DAC applications and highlights the importance of a sensitive analysis seeking for energy-efficient CO2 capture technology.
REFERENCES
[1] W.B. Abhinav Srinivas, Evaluation of Purolite® A110 and Lewatit® VP OC 1065 in a 1kg CO₂/day Fixed Bed DAC Unit, Proceedings of the 17th Greenhouse Gas Control Technologies Conference (GHGT-17), Canada, 2024.
[2] M.-Y.A. Low, D. Danaci, H. Azzan, R.T. Woodward, C. Petit, Measurement of Physicochemical Properties and CO2, N2, Ar, O2, and H2O Unary Adsorption Isotherms of Purolite A110 and Lewatit VP OC 1065 for Application in Direct Air Capture, Journal of Chemical & Engineering Data 68(12) (2023) 3499-3511. https://doi.org/10.1021/acs.jced.3c00401.