2025 AIChE Annual Meeting
(135c) Microfluidically Engineered Platform to Enable Immune Phenotyping on the Single-Cell Scale
Author
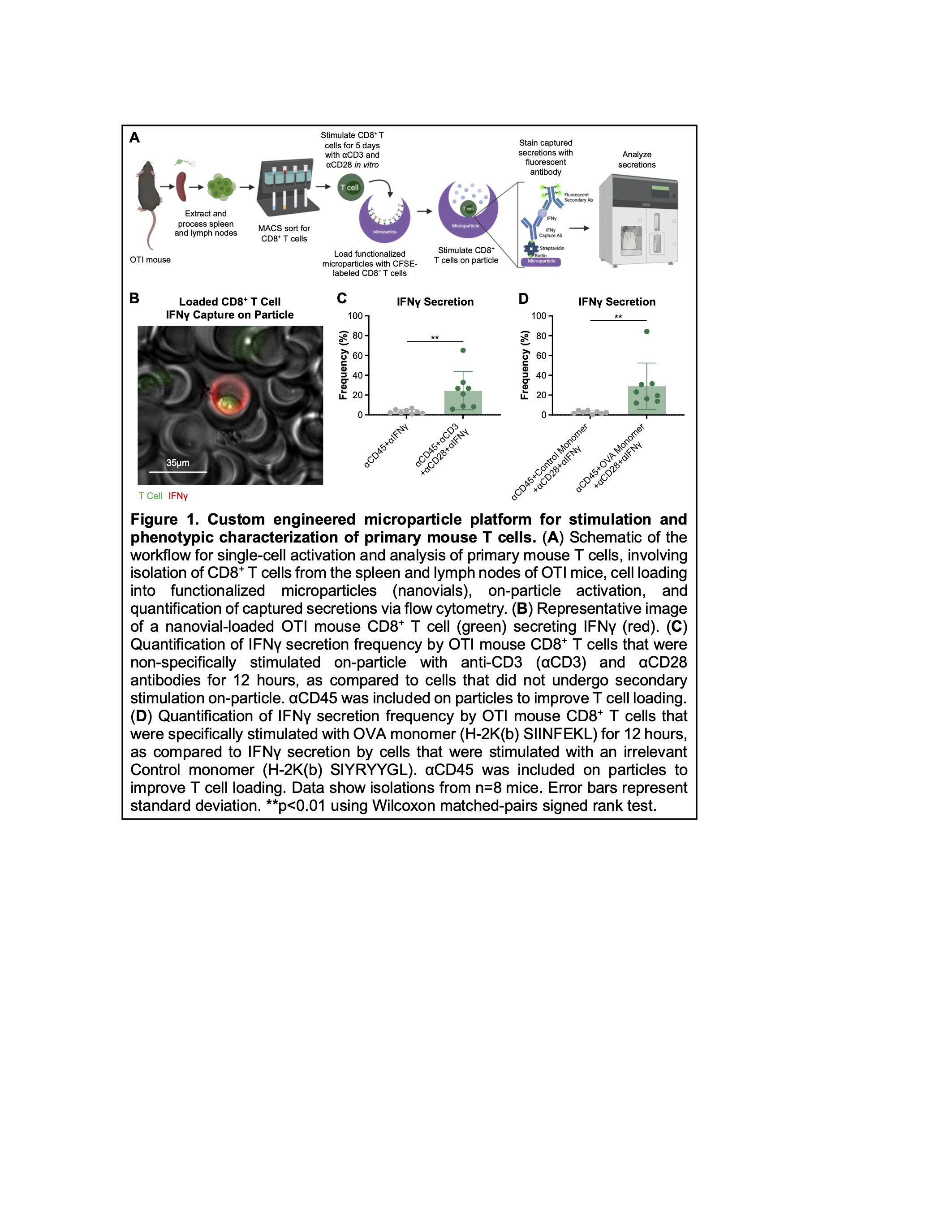
We first established the capacity for both nonspecific and antigen-specific stimulation of mouse CD8+ T cells within nanovials, by coating either anti-CD3 and anti-CD28 antibodies or peptide-loaded major histocompatibility complexes, respectively, on particles (Figure 1B-D). We further demonstrated the ability to multiplex the capture of protein secretions, simultaneously quantifying the amount of interferon gamma (IFNg) and tumor necrosis factor alpha (TNFa) secreted by individual mouse T cells. We also demonstrated the capacity to sort viable T cells based on their cytokine secretion profiles, which is highly relevant for engineered cell manufacturing. Finally, we leveraged our platform to profile the phenotype of resting mouse CD8+ T cells that would go on to secrete IFNg following stimulation within nanovials. Interestingly, we found that cells that expressed the activation markers CD44, CD62L, and/or CD127 as well as cells that expressed the exhaustion markers T cell immunoglobulin and mucin domain 3 (TIM3), lymphocyte activation gene 3 (LAG3), killer cell lectin-like receptor G1 (KLRG1), and/or programmed cell death protein 1 (PD-1) were more likely to secrete IFNg in response to either nonspecific or antigen-specific stimulation. Building on these findings, we are developing workflows to explore cell heterogeneity and identify the phenotypic correlates of function for other immune cell types. In tandem, we are evaluating the cytotoxic and effector activities of cells that secrete high levels of cytokines following stimulation in order to directly link therapeutically relevant functional outcomes to surface protein expression profiles.
Overall, the nanovial workflow we developed offers unprecedented insight into immune biology at the single-cell level and harbors great potential to guide the design of next-generation immunotherapies. Moreover, the compatibility of our platform with standard cell sorters allows enables its widespread use in the scientific community for a host of applications in molecular and cellular engineering.
Reference
- de Rutte, J. et al. Suspendable Hydrogel Nanovials for Massively Parallel Single-Cell Functional Analysis and Sorting. ACS Nano 16, 7242–7257 (2022).