2025 AIChE Annual Meeting
(307b) Microfluidic Detection Tool for Anaplasma Spp. Infections Via Electrokinetics
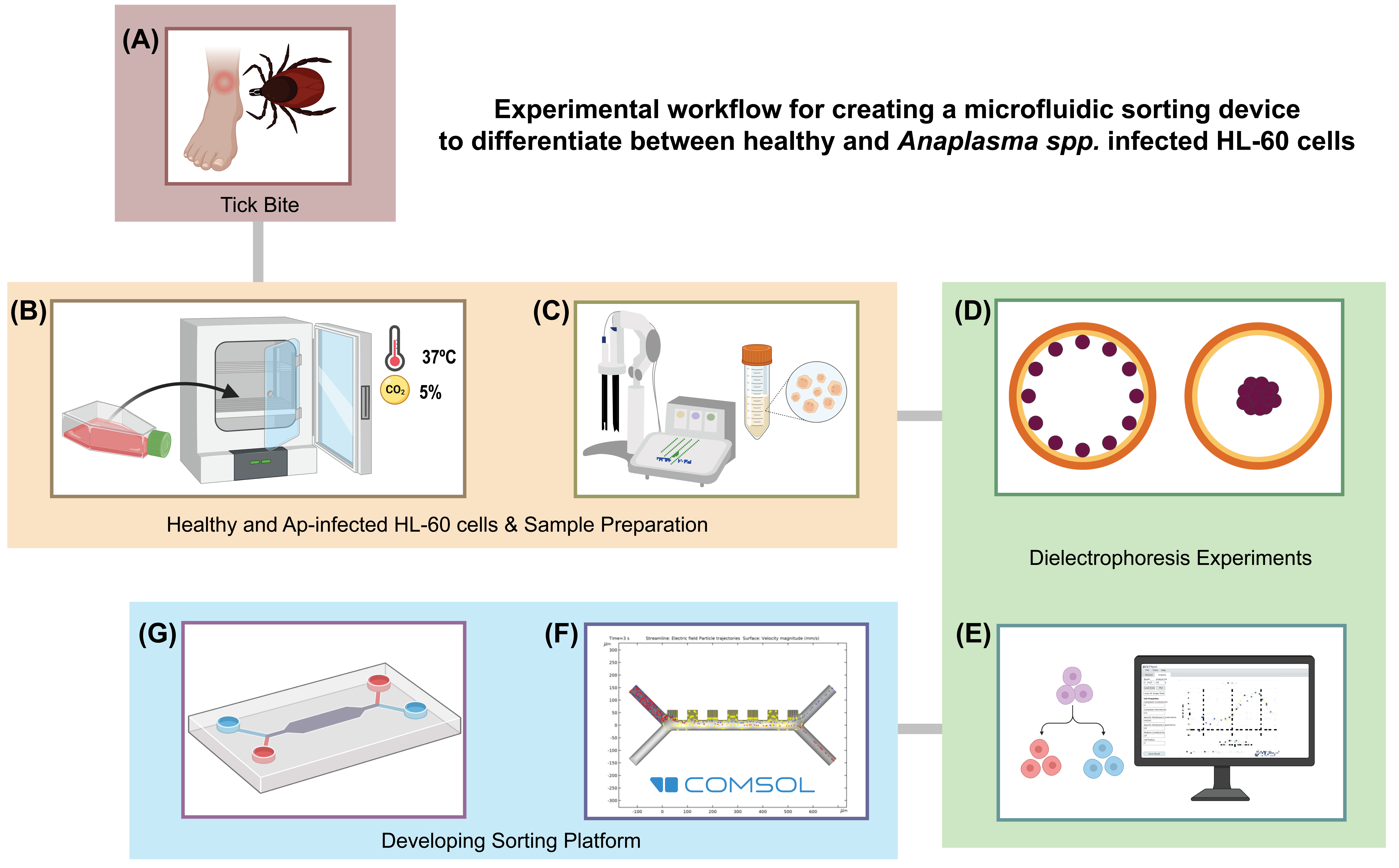
Figure 1: Experimental workflow for creating a microfluidic sorting device to differentiate between healthy and Anaplasma spp. infected HL-60 cells. (A) Anaplasmosis is transmitted through a tick bite, (B) Healthy and Anaplasma spp. infected HL-60 cells are cultured in an incubator, (C) A buffer medium containing 0.8% sucrose and 0.3% dextrose is prepared, with a conductivity range of 0.01-0.05 S/m, adjusted using 1x PBS, (D) The crossover frequency, where cells neither move toward nor away from the electrodes, is determined using the dielectrophoresis technique, (E) Data from healthy and infected cells are compared, (F) The COMSOL platform is used to simulate the sorting device based on the experimental data and (G) The optimized sorting platform is 3D printed using a CADWorks 3D printer.
References
[1] A. Hojgaard et al., "Geographic variation in the distribution of Anaplasma phagocytophilum variants in host-seeking Ixodes scapularis nymphs and adults in the eastern United States elucidated using next generation sequencing," Ticks and Tick-borne Diseases, vol. 15, no. 5, p. 102360, 2024/09/01/ 2024, doi: https://doi.org/10.1016/j.ttbdis.2024.102360.
[2] J. Yang et al., "Evaluation of different nested PCRs for detection of Anaplasma phagocytophilum in ruminants and ticks," BMC Veterinary Research, vol. 12, 02/24 2016, doi: 10.1186/s12917-016-0663-2.
[3] R. Oladokun, E. Adekanmbi, M. Ueti, and S. Srivastava, "Dielectric characterization of Babesia bovis using the dielectrophoretic crossover frequency," ELECTROPHORESIS, vol. 44, no. 11-12, pp. 988-1001, 2023/06/01 2023, doi: https://doi.org/10.1002/elps.202200263.
[4] N. F. Doost, S. D. R. Yaram, K. Wagner, H. Garg, and S. K. Srivastava, "Bioelectric profiling of Rickettsia montanensis in Vero cells utilizing dielectrophoresis," Journal of Biological Engineering, vol. 19, no. 1, p. 18, 2025/02/18 2025, doi: 10.1186/s13036-025-00487-y.
[5] M. Park Jason et al., "An Anaplasma phagocytophilum T4SS effector, AteA, is essential for tick infection," mBio, vol. 14, no. 5, pp. e01711-23, 2023, doi: 10.1128/mbio.01711-23.
[6] K. F. Hoettges et al., "Ten-Second Electrophysiology: Evaluation of the 3DEP Platform for high-speed, high-accuracy cell analysis," (in eng), no. 2045-2322 (Electronic).