2025 AIChE Annual Meeting
(344d) Microbial Rock Weathering for CO2 Sequestration: Overcoming Process Limitations with Genetic Engineering
Authors
Eq. 1. MgFeSiO4 + 4H2O + 4CO2 --> Mg2+ + Fe2+ + H4SiO4 + 4HCO3-
Olivine dissolution in seawater is governed by iron, which is present in nearly all minerals. In aerobic conditions at circumneutral pH, iron spontaneously oxidizes and precipitates back on the mineral surface, severely restricting mineral dissolution.2 The insolubility of iron also presents a challenge for microorganisms, for which iron is an essential micronutrient. To overcome this, bacteria secrete siderophores—a diverse class of secondary metabolites that chelate and solubilize ferric iron. Bacteria were shown to acquire iron from olivine using siderophores.3 Siderophores have also been shown to accelerate the olivine dissolution at neutral pH.4
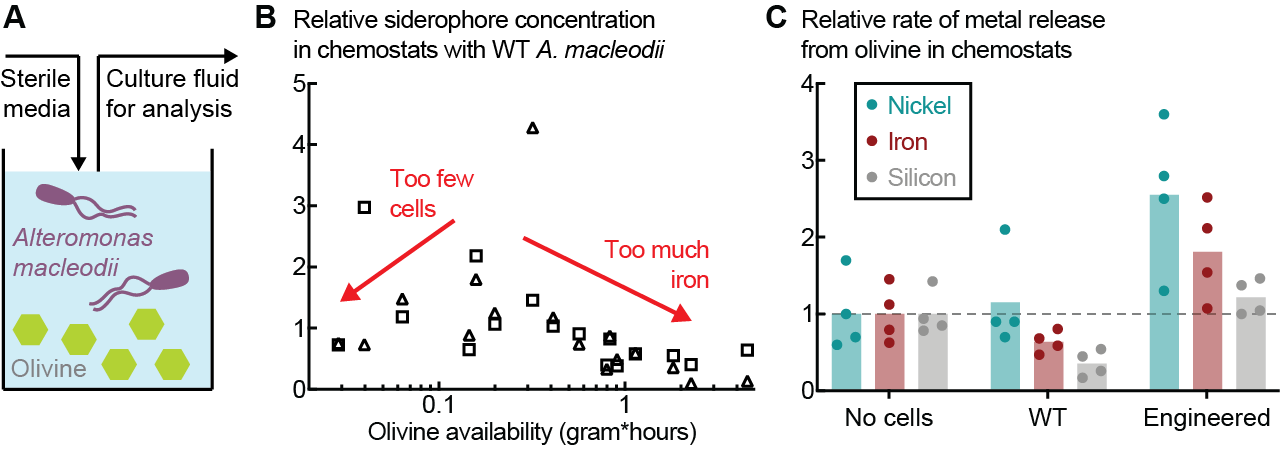
We developed a novel experimental platform to assess the impact of microorganisms on dissolution of olivine. We grew the ubiquitous marine bacterium Alteromonas macleodii in continuous cultures (chemostats) with olivine sand (Fig. 1A). This enables, for the first time, precise coupling of microbial physiology with geochemical measurement of mineral dissolution. We used this platform to determine process conditions in which bacteria can and cannot continuously accelerate olivine dissolution.
We observed siderophore production only during iron-limiting growth, restricting the quantity of olivine that can be processed by cells (Fig. 1B). To overcome this limitation, we genetically engineered A. macleodii for constitutive production of siderophores. Engineered strains produce >100x more siderophore than the base strain, independent of process conditions and iron availability. This increase conferred a 2-3x improvement in olivine dissolution rate in continuous cultures (Fig. 1C).
This work is the first application of synthetic biology and genome engineering for mineral weathering. The experimental platform reported here will enable further optimization of strains for siderophore-mediated mineral dissolution, discovery of additional biological weathering mechanisms, and design of pilot scale mineral bioreactors. We envision deployment of contained, wastewater-scale, bioreactors for safe, accelerated mineral dissolution and carbon sequestration on the decadal timescale.
- Renforth, P. & Henderson, G. Assessing ocean alkalinity for carbon sequestration. Reviews of Geophysics 55, 636–674 (2017).
- Fuhr, M. et al. Kinetics of Olivine Weathering in Seawater: An Experimental Study. Frontiers in Climate 4, 39 (2022).
- Van Den Berghe, M., Merino, N., Nealson, K. H. & West, A. J. Silicate minerals as a direct source of limiting nutrients: Siderophore synthesis and uptake promote ferric iron bioavailability from olivine and microbial growth. Geobiology 19, 618–630 (2021).
- Torres, M. A., Dong, S., Nealson, K. H. & West, A. J. The kinetics of siderophore-mediated olivine dissolution. Geobiology 17, 401–416 (2019).