2025 AIChE Annual Meeting
(636a) Methods to Purify, Preserve, and Recycle Polyolefins Using Green Solvents and Water
Authors
Plastics processing with affordable, environmentally benign solvents can solve these problems. Of which, the most abundant and least toxic solvent of interest is water. As is well-known, room temperature water is a non-solvent for nearly all engineering plastics, meaning it is useless for polymer recycling. Processing polymers at extrusion conditions in liquid water similarly has little effect and instead results in brittle blends. A common thread in chemical recycling literature, however, is the use of near and supercritical water as a solvent for destruction of polymers into fuels or monomers. This drastic change in polymer behavior between extrusion conditions (200 °C) to supercritical (374 °C) can be accounted for by the change properties of water. Specifically, the dielectric constant of water changes dramatically with temperature, to the extent that hydrocarbons are miscible with near and supercritical water whereas salt becomes immiscible in it. This change in solubility can be captured through careful selection of operating conditions to selectively swell hydrocarbons such as plastics without depolymerization.
We hypothesize that between these two extremes in solubility conditions, there exists a previously unevaluated regime for plastics recycling. Operating at an intermediate temperature between those required for deconstruction and those at which no blending occurs, water expands the polymer melts, allowing them to mix with one another. This melt expansion can also enable the extraction of additives, as the expanded polymers will be highly mobile, and many additives will be water soluble under these conditions. This process can be termed “chemi-mechanical recycling” as the resulting product bridges the industrial gap between upgraded pyrolyzed fuels and underperforming mechanical recyclate.
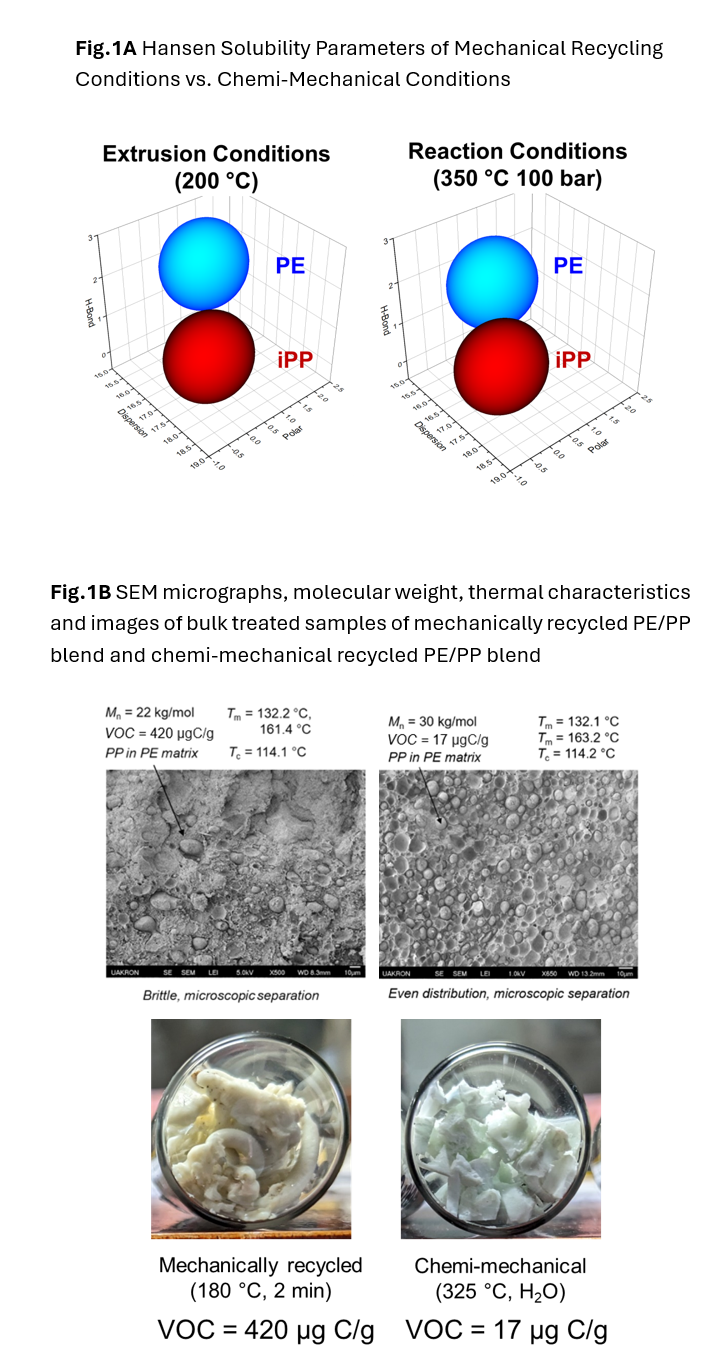
Two of the most commonly produced plastics, polyethylene (PE) and polypropylene (PP), account for over half of all plastics produced but have very low recycling rates. Despite similar monomers, these two polymers are immiscible and blending by traditional means results in products that suffer from poor interfacial adhesion and phase separation. Using chemi-mechanical recycling, we can overcome this adhesion barrier by operating at temperatures above extrusion conditions, PE and PP become more soluble with each other. This can be visualized using Hansen solubility parameters (HSPs) as seen in Figure 1a. At extrusion conditions, the spheres of PE and PP do not overlap, indicating poor solubility. However, at chemi-mechanical conditions, PE and PP become more soluble with each other. The accompanying SEM micrographs in Figure 1b provide an experimental basis for these phenomena. Under extrusion conditions, the resulting product maintains a high molecular weight but suffers from poor interfacial adhesions and retains a high level of volatile organic compounds (VOCs). Comparatively, the chemi-mechanical recyclate shows a uniform dispersion of PP droplets within a PE matrix and a high retention of molecular weight. This further shows that at chemi-mechanical conditions, water can promote intermixing of PE and PP due to swelling effects.
An area of ubiquitous importance in plastics recycling is the removal of additives and VOCs. Many plastics have a high number of VOCs which cannot be removed via mechanical recycling thus limiting the applications of this recyclate. As seen in Figure 1b, the mechanically recycled blend visually appears more degraded and retains a high VOC concentration of 420 µgC/g. Comparing to the chemi-mechanical sample in Figure 1b, the water blend is lighter and appears cleaner which translates to lighter color values and a 96% reduction in VOCs. As plastic waste contains a diverse stream of additives however, additional analysis is underway to further improve the extractive capabilities of chemi-mechanical recycling. This significant reduction in both color and organic content indicates that chemi-mechanical recycling is able to act as an extractive process, suitable for purifying post-consumer waste.
The chemi-mechanical recycling process has proven to be an effective alternative to mechanical recycling as it can maintain molecular weight, achieve micro-scale blending and extract VOCs and colorants. These successes warrant investigating the economic and environmental impacts of the water-based process. Economic assessment indicates that chemi-mechanical is cost-competitive with a minimum selling price calculated as $1.34 per kg of recyclate – which is comparable to the cost of virgin PE at 0.86-1.79 USD/kg. Emissions analysis further indicates the industrial viability of chemi-mechanical recycling with an estimated environmental impact of 0.47 kg CO2 eq per kg plastic calculated, which is comparable to the emissions associated with mechanical recycling (0.48 kg CO2 eq per kg plastic). These are conservative estimates as the associated scale assumed in these calculations was 3 tons per day which accounts for just 1% of total daily plastic waste generation in the US. The costs and emissions projected in this student encourage follow-on development considering the conservative scale assumed.
In summary, treatment of a PP-PE mixture in water near its critical point produces an intimately mixed blend which retains a high molecular weight and its original melting characteristics. The extractive nature of the aqueous process reduces volatile organic compounds by 96% and reduces the color of the recycled resin. Chemi-mechanical recycling was found to be economically and environmentally competitive with existing technologies, encouraging further process development and scale-up. The results presented here establish a previously undiscovered regime that combines chemical and mechanical recycling as a new approach to transform mixed plastic waste streams into a purified and compatibilized product.