2025 AIChE Annual Meeting
(319e) Methane Pyrolysis over Carbon Catalysts for Hydrogen Production: Impact of Carbon Deposition on Carbon Electrical Conductivity and Polyaromatic Hydrocarbon Formation
Authors
Lukas Buelens, Ghent University
Manly Callewaert, Ghent University
Parviz Yazdani, Ghent University
Joris Thybaut, Ghent University
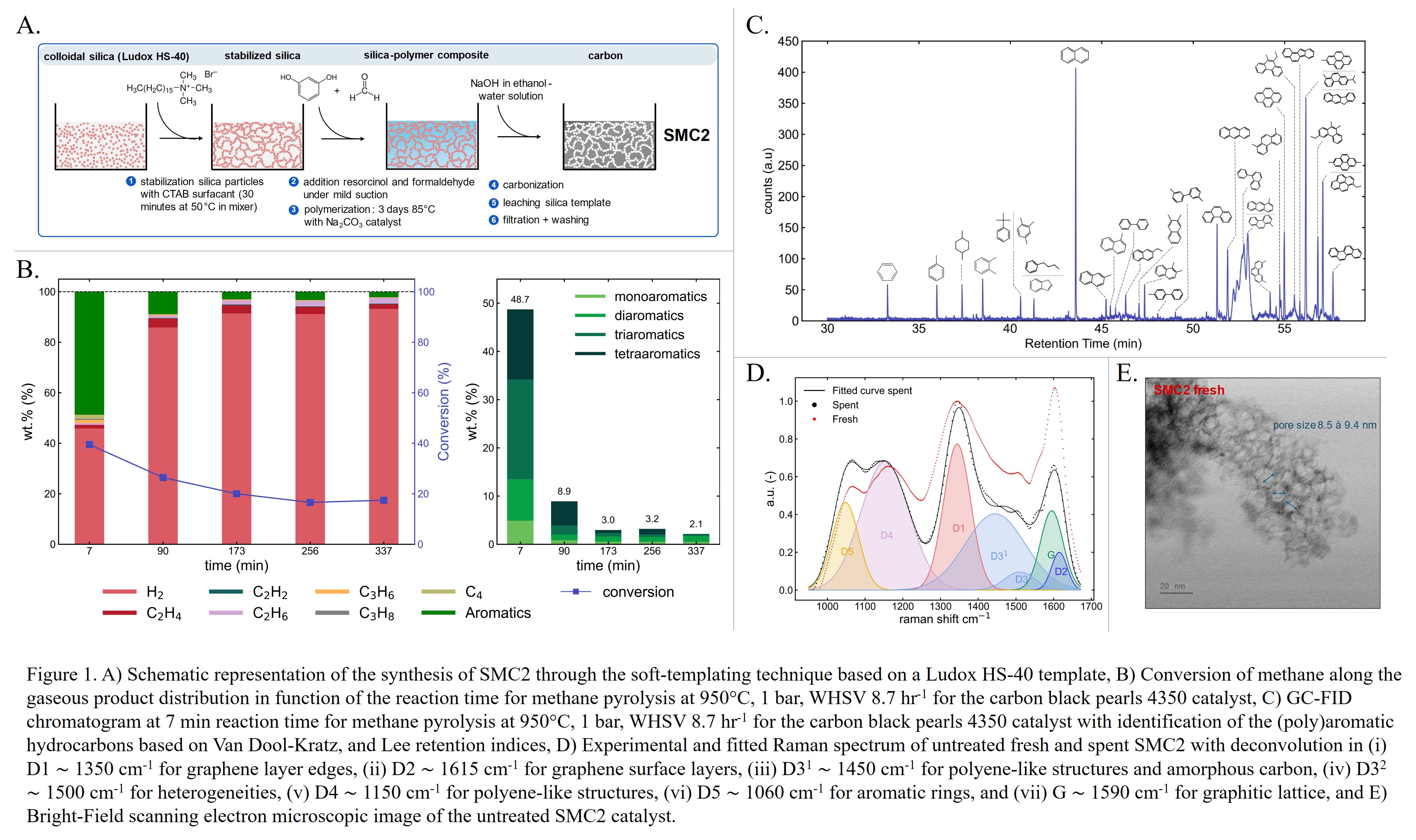
Methane pyrolysis is a promising technology for low-cost, low-emission hydrogen (H2) production by decomposing methane into H2 and value-added carbon. Coking-induced deactivation, a major challenge in metal-based, catalysts can be alleviated using carbon catalysts. However, the impact of carbon deposition on the structural changes in carbon catalysts and corresponding product distribution remain unclear. The formation of polyaromatic hydrocarbons (PAHs) that cause process equipment fouling and possible downtimes, is of particular importance. Moreover, the impact of carbon deposition on the electrical properties of carbon catalysts, key for their potential application in electrified reactor configurations based on Joule- or inductive heating, is not yet understood. In this work, carbon black pearls 2000, carbon black pearls 4350, and SMC2 ordered mesoporous carbon based on the soft-templating technique on a colloidal silica template have been characterized (N2-physisorption, Raman, CHN/O elemental analysis, and BF-STEM) to determine the structure of the carbon deposits after methane pyrolysis in a fixed-bed reactor at 950°C. SMC2 exhibited the highest initial H2 yield attributed to its high specific surface area (1243 m²·gcat,o-1) and initial oxygen content (7.7 wt.%), but gradually deactivated through pore filling and blockages. Carbon black pearls 2000 demonstrated greater stability, achieving the highest cumulative carbon yield (3.7 gc,produced·gcat,o-1). Carbon deposition led to the formation of graphitic domains around the primary carbon black particles, reducing structural heterogeneities and defects in the overall carbon framework. Additionally, its accumulation on the catalyst surface suppressed formation of PAHs, as identified by Van Dool-Kratz and Lee retention indices, decreasing from 47 wt.% to 2 wt.% after 5 hours of methane pyrolysis. Initially dominated by tetra-aromatics, the PAH composition shifted towards monoaromatics and diaromatics, with naphthalene being the major product. Last, impedance spectroscopy revealed a decrease in the catalyst's electrical resistivity upon carbon deposition, attributed to reduced porosity and a denser structural framework.