2025 AIChE Annual Meeting
(460e) Methane Oxidation over Iridium Dioxide Catalysts
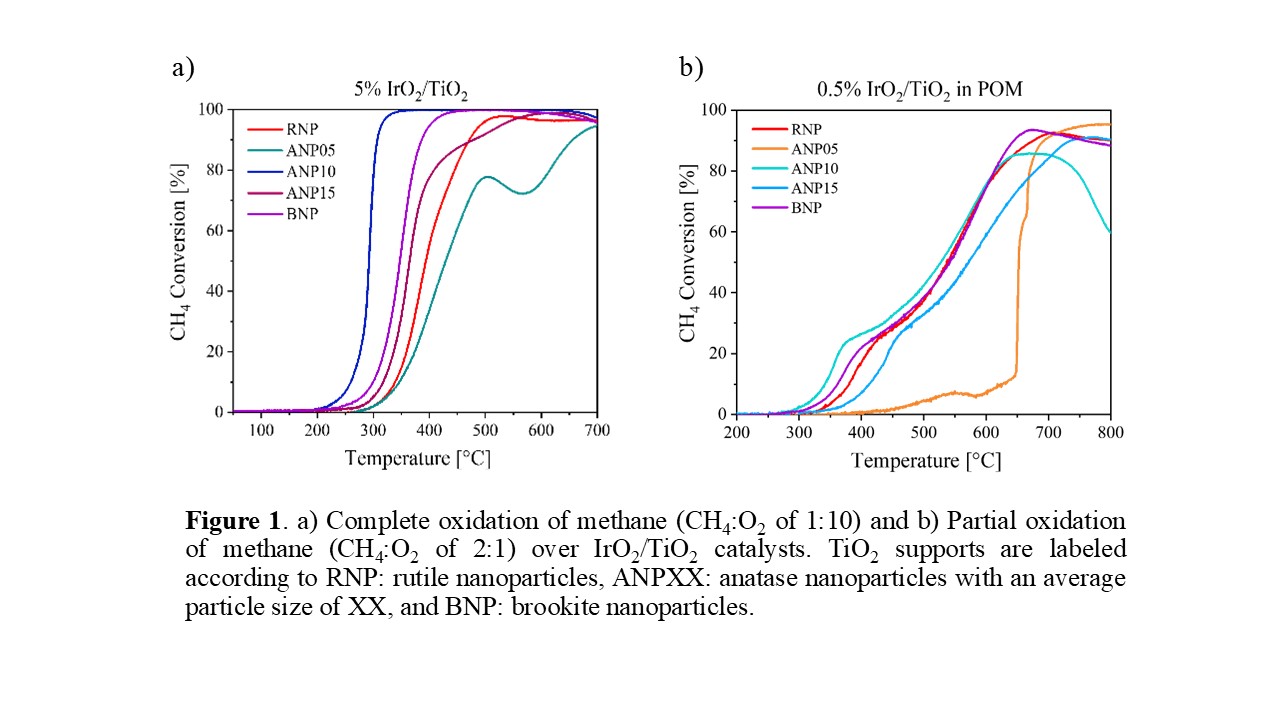
The (110) surface facet of rutile IrO2 can activate methane below room temperature, but the active sites on this surface are blocked in the presence of very low concentrations of gas phase oxygen. Selective transformations of methane to higher value chemicals over IrO2-based catalysts are therefore challenging despite the ability to activate methane at low temperatures. To gain a better understanding of IrO2-based catalysts our group has investigated IrO2 supported on TiO2 with different structures and particle sizes. Under complete oxidation conditions (CH4:O2 of 1:10), both the structure and the particle size of the TiO2 nanoparticle support influence the catalytic activity. Catalyst characterizations reveal that on anatase TiO2 small IrO2 nanoparticles are visible, while a thin layer of IrO2 is observed on both brookite and rutile TiO2 nanoparticles. The IrO2-TiO2 interactions result in more electron-rich IrOx species on anatase TiO2 compared with the other TiO2 supports. This appears to be beneficial in the complete oxidation of methane, as the IrO2 supported on anatase TiO2 is more active compared with IrO2 supported on brookite TiO2, and the least active catalyst is IrO2 supported in rutile TiO2 (Figure 1a). However, the activity of these catalysts is also influenced by the particle size of the support. The best performing catalyst is IrO2 supported on anatase TiO2 with an average particle size of 10 nm, and IrO2 on 5-nm or 15-nm anatase TiO2 exhibit inferior activity. In fact, the worst performing catalyst of all IrO2/TiO2 tested is the IrO2 supported on 5-nm anatase TiO2, likely due to an unstable support. Under partial oxidation conditions (CH4:O2 of 2:1), the influence of TiO2 structure on the activity is less significant (Figure 1b). This is due to the reaction pathway, which involves complete combustion while oxygen is present, followed by dry and steam reforming of methane.