2025 AIChE Annual Meeting
(360b) Metastable CoCu2O3 Nanocrystals from Combustion-Aerosols for Molecular Sensing and Catalysis
Authors
Tiago Elias Abi-Ramia Silva, ETH Zurich
Edoardo Consogno, ETH Zurich
Frank Krumeich, Particle Technology Laboratory, ETH Zurich
Andreas Güntner, ETH Zürich
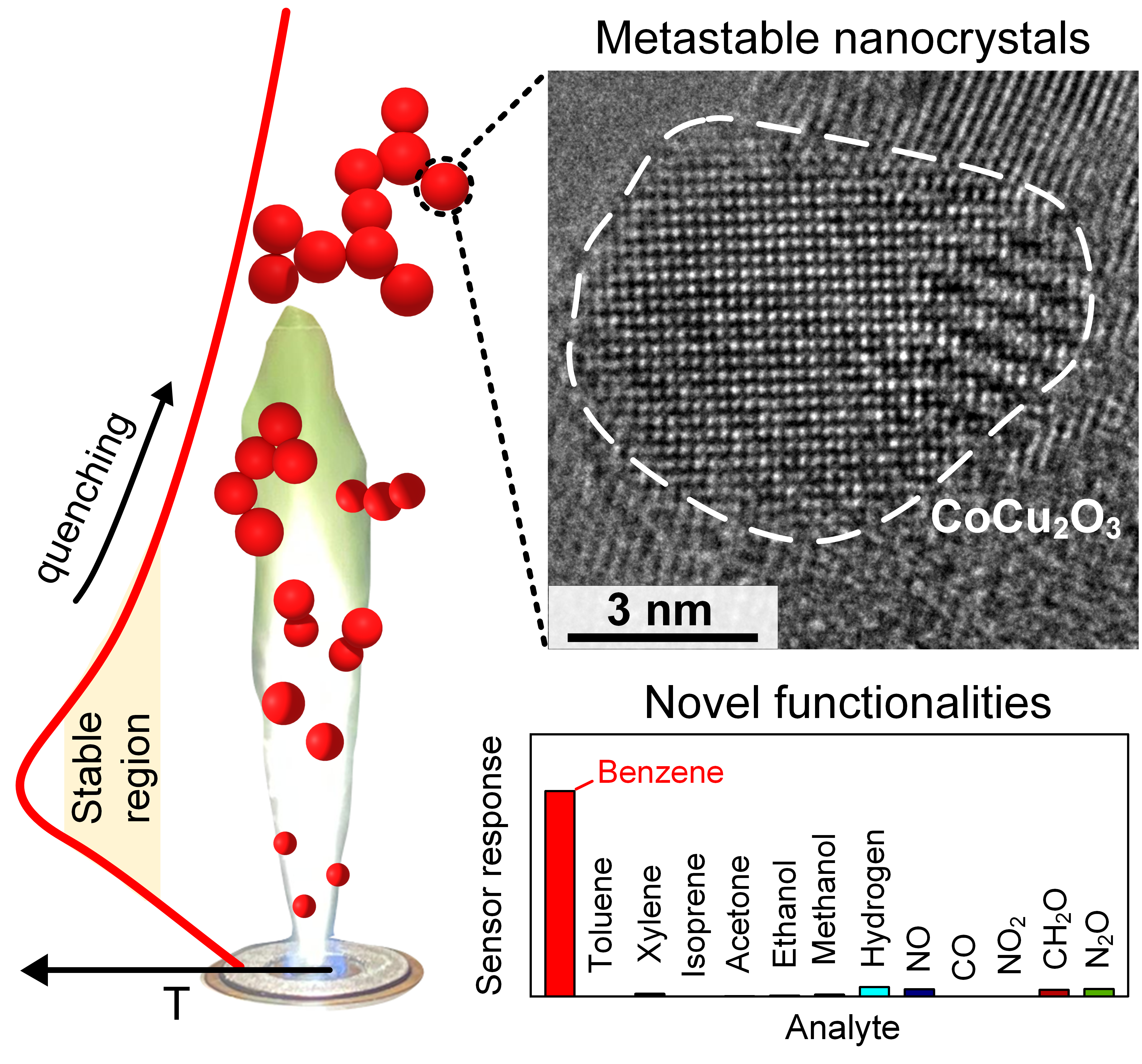
Metastable nanostructures are kinetically trapped in local energy minima featuring intriguing surface and material properties. To unleash their potential, there is a need for non-equilibrium processes capable of stabilizing a large range of crystal phases outside thermodynamic equilibrium conditions by closely and flexibly controlling atomic reactant composition, spatial temperature distribution and residence time [1]. Here, the capture of metastable pseudo-binary metal oxides at room temperature is demonstrated with scalable combustion-aerosol technology [2]. By a combination of X-ray diffraction, electron microscopy and on-line flame characterization, the occurrence of metastable CoCu2O3 is investigated with controlled crystal size (4 – 16 nm) over thermodynamically favored CuO and Co3O4. Immediate practical impact is demonstrated by exceptional sensing and stable catalytic performance for air pollutant detection, e.g., quantifying benzene down to 15 parts-per-billion (ppb) in the co-presence of up to 5000 ppb interfering molecules including alcohols, terpenes and, most impressively, chemically similar toluene and xylene. Additionally, secondary phases can be loaded on such metastable nanostructures to access novel materials promising for actuators, energy storage or solar cells.
References
[1] Pratsinis, S.E., Prog. Energ. Combust. Sci. 1998, 24, 197 – 219.
[2] D’Andria, M; Elias Abi-Ramia Silva, T.; Consogno, E.; Krumeich, F. & Güntner, A.T., Adv. Mater. 2024, 36, 2408888.