2025 AIChE Annual Meeting
(116e) Metal-Support Interfaces Overcome Reactivity Constraints in Oxygen Electrocatalysis
Authors
Tej Choksi - Presenter, Nanyang Technological University
Asmee Prabhu, Nanyang Technological University
Kah Meng Yam, Nanyang Technological University
Bryan Chak Sing Lee, Nanyang Technological University
Lavie Rekhi, NTU Singapore
Luan Q. Le, Nanyang Technological University
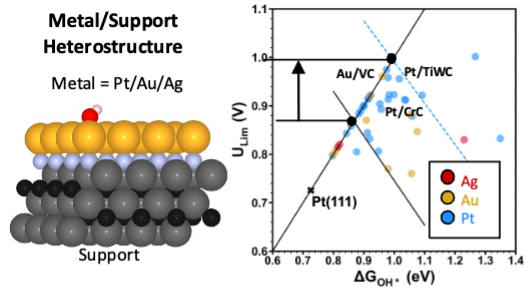
Platinum-based catalysts are widely used for the Oxygen Reduction Reaction (ORR) in fuel cells, metal-air batteries, and electrolyzers due to their excellent electrocatalytic activity and stability. However, the cost and scarcity of platinum have led to efforts in alloying platinum with other transition metals and nano-structuring the Pt surface. Moreover, the maximum reactivity of Pt-based catalysts is constrained by bond order conservation-based scaling relations. Using periodic density functional theory calculations, we assessed the mechanical, thermodynamic, and electrochemical stability of 200+ supported metal catalysis. Their ORR catalytic activity was evaluated using a limiting potential analysis. The OOH* vs OH* scaling relation deviates from the bond order conservation slope of 1 (seen across unsupported metals, alloys, and single-atom catalysts). This deviation in the slope results in an upward shift to the ORR limiting potential volcano peak by >0.15 V, relative to unsupported metal catalysts. These deviations in slopes are attributed to modulations in electronic metal-support interactions and interfacial strain. Our computational analysis explains the superior stability and enhanced performance of experimentally studied Pt/TiWC catalysts, thus revalidating prior experiments. Furthermore, we discover 18 other supported metal catalysts with higher limiting potentials than Pt (111), expanding this library of supported metal catalysts. Within this library, Pt/CrC is the most reactive towards ORR with a limiting potential at 0.93 V. Moreover, this library contains Au-based catalysts that exceed the limiting potential of Pt(111), in contrast to the otherwise unreactive nature of unsupported Au. Charge transfer and localized dipoles at the metal/support interface are key drivers of improved stability and reactivity. The concepts introduced in this work posits that engineering the support of ORR catalysts can: (1) stabilize low-dimensional metal films, (2) enhance their reactivity, and (3) minimize precious metal loading, thereby emerging as a powerful strategy for overcoming reactivity constraints in oxygen electrocatalysis.