2025 AIChE Annual Meeting
(51d) Metal Oxide Mobility in Zeolite: Impact on Hydrocarbon Pools and Its Inhibition Via Silicalite-1 Coating during CO? Hydrogenation
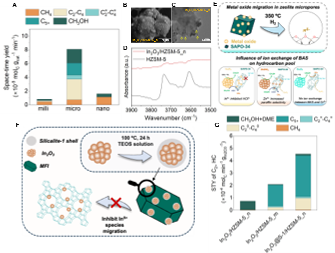
We show that HC space-time-yield (STY) can be enhanced ~8 by increasing proximity of redox sites and BAS from milliscale to microscale on In2O3 and HZSM-5 system. However, increasing the proximity further to nanoscale caused the migration of In2O3 into HZSM-5 micropores and subsequent ion exchange of BAS with Inδ+, inhibiting the acidity of HZSM-5 and its C-C coupling ability. This led to our hypothesis that metal oxide mobility dictates i) the likelihood of BAS exchange with cations, and ii) affects HCP. Hence, we probed C3/C2 HC ratio and paraffin-to-olefins (P/O) ratio during reaction. We revealed that while Inδ+ inhibited HCP yielding C3/C2~0 and P/O~0, Znδ+ enhanced hydrogen-transfer yielding ~5 higher P/O ratio and decreased olefin selectivity, compared to its microscale proximity admixture where ion exchange did not occur. We then aimed at coating metal oxides with S-1 shell. The performance of S-1 coated In2O3 (In2O3@S-1) at nanoscale proximity with HZSM-5 (In2O3@S-1/HZSM-5_n) exhibited ~5 higher yield of C2+ HC as compared to In2O3/HZSM-5_n, indicating ion exchange of BAS with Inδ+ was likely inhibited. Additionally, In2O3@S-1/HZSM-5_n exhibited ~2 higher C5+ HC than microscale proximity (In2O3/HZSM-5_m), indicating efficient transfer of CH3OH favored methylation, enhancing C5+ selectivity at nanoscale proximity.