2025 AIChE Annual Meeting
(714c) Mechanistic Insights into the Concentration-Dependent Anomalous Diffusion and Aggregation of Monoclonal Antibodies
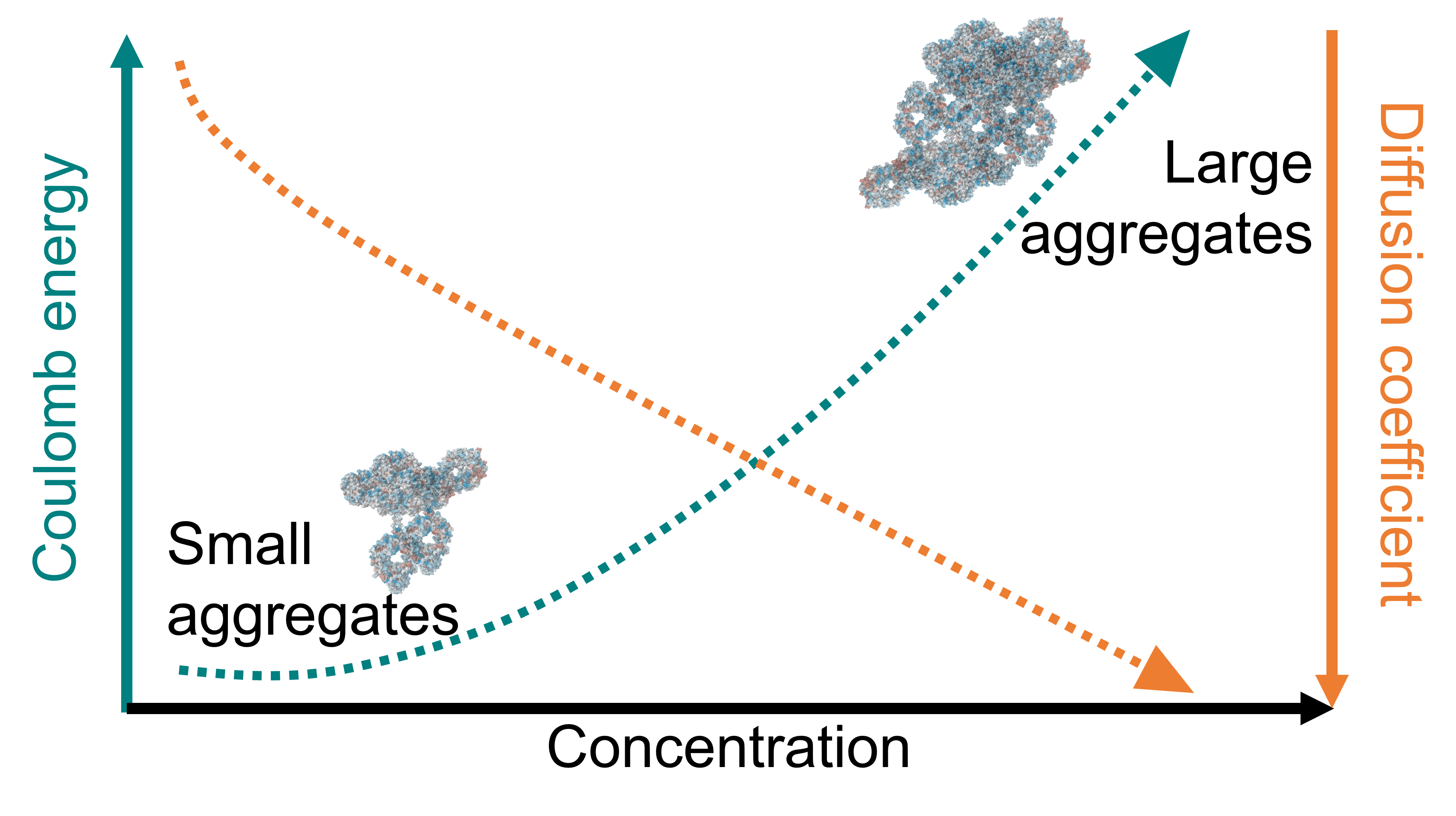
The formulation, storage, transportation, and subcutaneous administration of highly concentrated monoclonal antibody (mAb) solutions present notable challenges, primarily due to the high solution viscosity and low diffusion of the antibody molecules in crowded environments. These difficulties often arise from antibody self-association, which can subsequently lead to aggregation. In this study, we utilized a dissipative particle dynamics (DPD)-based coarse-grained computational model to explore the diffusion characteristics of IgG1 antibodies in aqueous solutions at varying antibody concentrations (10 to 400 mg/mL) and ionic strengths (15 and 32 mM NaCl). Interaction parameters for the coarse-grained simulations were calibrated by aligning the computed structure factor with existing experimental and computational literature data. Our analysis revealed a transition from Fickian diffusion at lower antibody concentrations (10 and 25 mg/mL) to anomalous diffusion at higher concentrations (≥50 mg/mL). This anomalous diffusion persisted for approximately 0.33 to 0.4 μs, after which diffusion behavior reverted to a Fickian regime. We observed a significant linear relationship between diffusion properties (diffusion coefficient D, and anomalous diffusion exponent α) and aggregate content, which itself correlated closely with the strength of Coulombic interactions among antibody molecules. Mechanistic investigation identified electrostatic attractions between complementary charged regions of antibody molecules as a critical factor promoting aggregation in crowded, high-concentration conditions. The continued action of Coulombic attraction facilitated the progressive formation of larger antibody aggregates, highlighting the complex interplay of molecular interactions in mAb formulations.