2025 AIChE Annual Meeting
(661e) Mechanism of Ge-Sn Chemical Vapor Deposition and Surface Diffusion: The Critical Role of Radical Species
Authors
Vy Nguyen - Presenter, University of Oklahoma
Tyler DeBlieck, Iowa State University
Luke T. Roling, Iowa State University
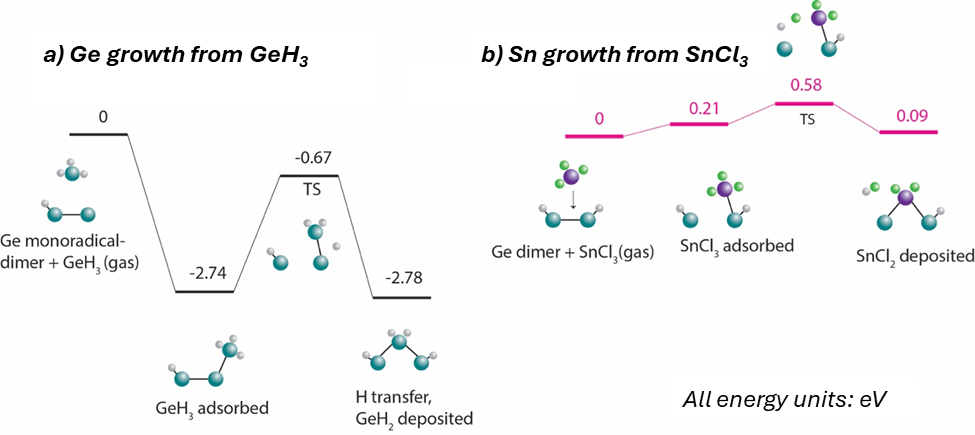
Germanium-tin (GeSn) alloys have attracted attention for use in next-generation optoelectronics, energy harvesting, and nanoelectronics materials due to their tunable direct bandgap and compatibility with existing Si-based technology. However, the low solubility of Sn in the Ge lattice presents substantial quality challenges related to the metastability of synthesized products. Relatively few details are known about the mechanism, motivating fundamental studies into atomic-scale mechanisms for growth to aid in identifying strategies for improving product quality. This presentation will share our insights into the growth mechanism of GeSn by chemical vapor deposition (CVD) on the hydrogen-passivated Ge(100) surface. Using density functional theory calculations, we reveal relatively low activation barriers for growth from germane (GeH4) or digermane (Ge2H6) and tin tetrachloride (SnCl4). The role of radical species is shown to be critically important in reducing activation energies, as Ge- and Sn-based radicals react readily in the gas phase to form GeHx and GeHxCly (x+y<4) species that generate dangling bonds on Cl-terminated and H-terminated surfaces to facilitate the further deposition of gas-phase reactants. Once deposited, the kinetics of surface diffusion for key species (GeH2, SnH2, and SnCl2) were analyzed under varying strain conditions. The results indicate that surface diffusion is highly strain-dependent, with diffusion pathways leading to the formation of Frenkel defects. Tin species exhibit higher surface mobility than germanium species, contributing to observed surface segregation and Sn out-diffusion. These insights into the GeSn growth mechanism and the kinetics of surface diffusion yield valuable strategies for optimizing synthesis conditions to produce high-quality GeSn materials for advanced optoelectronics applications.