2025 AIChE Annual Meeting
(403n) Mechanism and Parametric Effects of Multilayered Plastic Film Delamination
Authors
Protitee Paromita - Presenter, University of Massachusetts Lowell
Abigail Lynch, University of Massachusetts Lowell
Fuat Sakirler, University of Massachusetts-Lowell
Kalsoom Jan, University of Massachusetts Lowell
Grace Chen, University of Massachusetts Lowell
Hsi-Wu Wong, University of Massachusetts Lowell
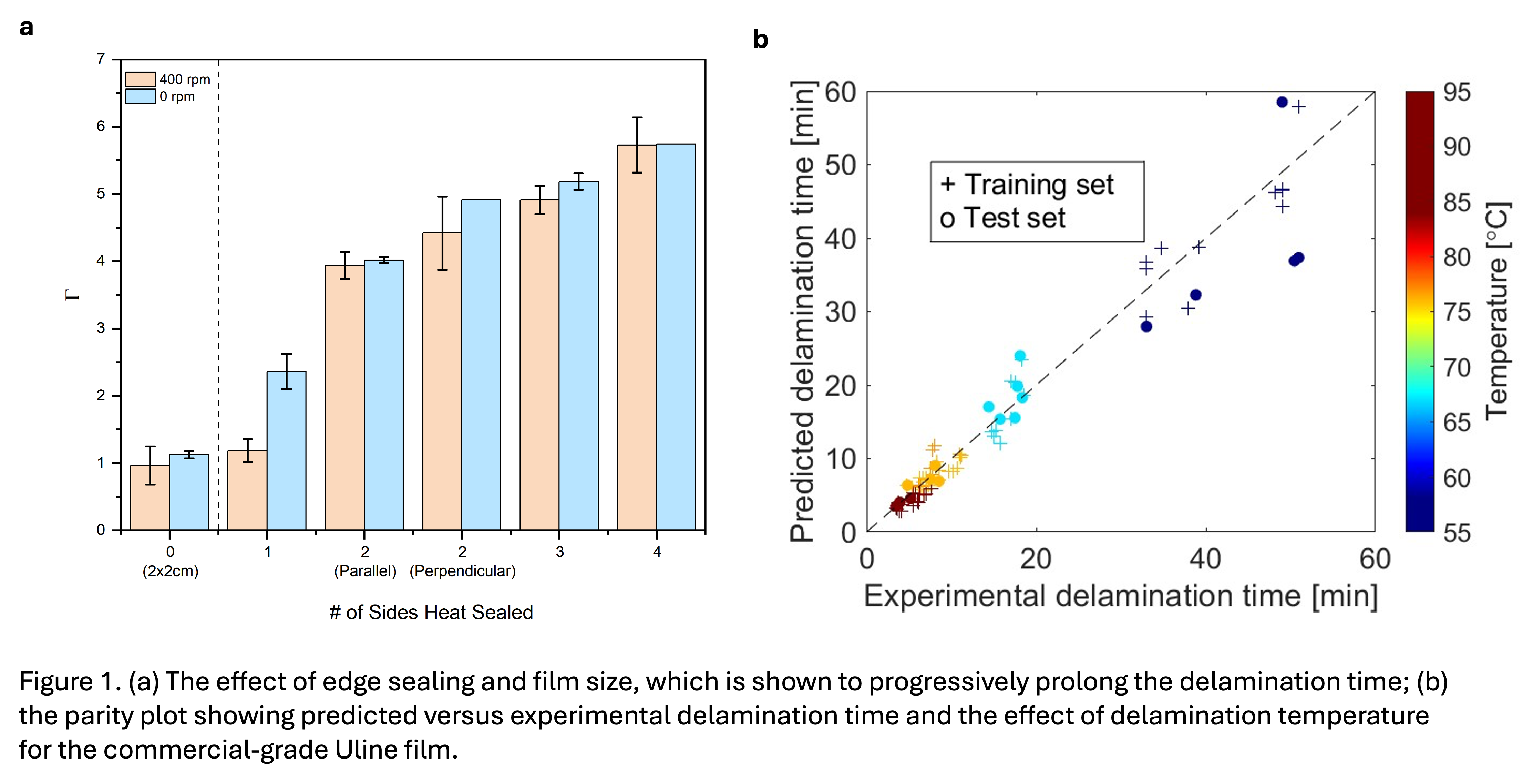
Plastics pose a large hurdle in promoting a circular economy due to challenges of recycling. This problem is further aggravated by the wide use of multilayered plastic films (MLPFs) for packaging, which cannot be recycled efficiently by conventional techniques. This study aims to investigate the mechanism and parametric effects of the delamination of two types of MLPFs using acetic acid. Heat sealing of film edges was first employed to lower the number of sides exposed to the acid for studying the diffusion mechanism. Films without heat-sealing treatment were found to have the shortest delamination time, while films with edges sealed were found to be significantly more difficult to delaminate (Fig. 1a), suggesting the primary delamination mechanism is diffusion of acid through the film edges into the interfaces between the layers. Following this discovery, parametric studies on the effects of perforation, temperature, and agitation speeds were conducted. A semi-empirical model was developed to describe the parametric effects of the delamination process at a wide range of experimental conditions. The model is capable of providing a robust representation of the two MLPFs tested, with predicted values well inline with the actual experimental data (Fig. 1b), demonstrating that it could be extended to other MLPFs of similar structures. Our work provides a framework for the design and development of optimal MLPF delamination processes for achieving efficient recycling.