2025 AIChE Annual Meeting
(28g) Maximizing Monomer Yield in Reductive Catalytic Fractionation of Woody Biomass through Solvent and Pressure Optimization
Authors
This study explores the potential of Reductive Catalytic Fractionation (RCF) for converting woody biomass into valuable aromatic monomers, with a particular focus on the role of different solvents and initial pressures. Lignin in woody biomass is a complex polymer that poses challenges for large-scale utilization. RCF offers a promising approach to breaking down lignin into monolignols, but a perfect solvent for lignin valorization has not yet been discovered. This study evaluates four solvents, i.e., tetrahydrofuran (THF), ethanol, methanol, and 1-pentanol, under varying pressure conditions to optimize monomer yields. Results indicate that while ethanol and methanol achieve higher pressures and monomer yields, THF and 1-pentanol present viable alternatives with lower operational pressures. The findings highlight the importance of solvent selection and pressure control in enhancing the efficiency and economic viability of lignin valorization processes. This research contributes to the development of sustainable methods for converting lignocellulosic biomass into biofuel precursors, with implications for improving process economics and environmental impact.
Introduction
Woody biomass, or lignocellulosic materials, represents a significant and yet underutilized resource of sustainable fuels and value-added chemicals. The annual production of the biomass is 181.5 billion tonnes, while out of which only 8.2 billion tonnes is being utilized. Wood comprises several chemical components, with cellulose, hemicellulose, and lignin being the primary constituents. These structural compounds are unevenly distributed within wood cell walls and vary significantly between species. 1 Among all the building block chemicals, lignin is the most important element to influencing the feasibility of a biorefinery. Lignin is a long-chain polymer composed of aromatic rings bonded together to form a complex, heterogeneous structure that contributes significantly to its structural stiffness and recalcitrant to bio/chemical degradation of the biomass. These aromatic rings, known as monomers or monolignols, include guaiacyl alcohol (G), syringyl alcohol (S), and p-coumaryl alcohol (H), etc. (Fig.1C). Such aromatic hydrocarbons are the second most significant component of crude oil, comprising 13 to 34% by weight. Despite its high industrial potential, large-scale lignin utilization remains challenging due to its complex polymeric structure. Several biorefinery techniques have been developed to increase the value of lignin products, e.g. through the production of monolignols.2
Reductive Catalytic Fractionation (RCF) is a promising process for breaking down lignin into valuable aromatic monomers. This process utilizes an external hydrogen source (either a solvent or hydrogen gas) and a heterogeneous catalyst to breakdown lignin into monomers, while an organic solvent with significant lignin solubility was applied to ensure the stability of the monolignols.3 THF is particularly effective due to its ability to dissolve lignin efficiently, facilitating the depolymerization process. However, a key challenge with using THF in RCF is its relatively low boiling point, which necessitates operating at higher pressures to achieve optimal monomer yields. This requirement increases operational costs, especially when scaling up for industrial applications. Additionally, the recovery and recycling of THF can be complex and energy-intensive, further impacting the economic viability of the RCF process.
The conversion efficiency of lignin to monolignols in RCF is highly dependent on reaction conditions, such as operating temperature and pressure. The reaction mechanism of RCF involves several key steps: solubilization of lignin, cleavage of ether bonds, and stabilization of the resulting monolignols. At different pressures, the mechanism can vary significantly, impacting the efficiency and yield of the process. At lower pressures, the solubilization of lignin may be less effective, leading to incomplete depolymerization and lower monomer yields. The reduced hydrogen availability can limit the stabilization of reactive intermediates, potentially resulting in repolymerization or side reactions that decrease the selectivity for desired monomers. Conversely, at higher pressures, the increased solubility of hydrogen enhances the reduction of lignin, facilitating more effective cleavage of ether bonds and stabilization of monolignols. This can lead to higher yields and selectivity for specific aromatic compounds. However, operating at high pressures also introduces challenges, such as increased safety risks and higher energy consumption, which can affect the economic viability of the process. The choice of solvent and catalyst, along with precise control of pressure conditions, is crucial to optimizing the RCF mechanism for efficient lignin valorization.
Methodology
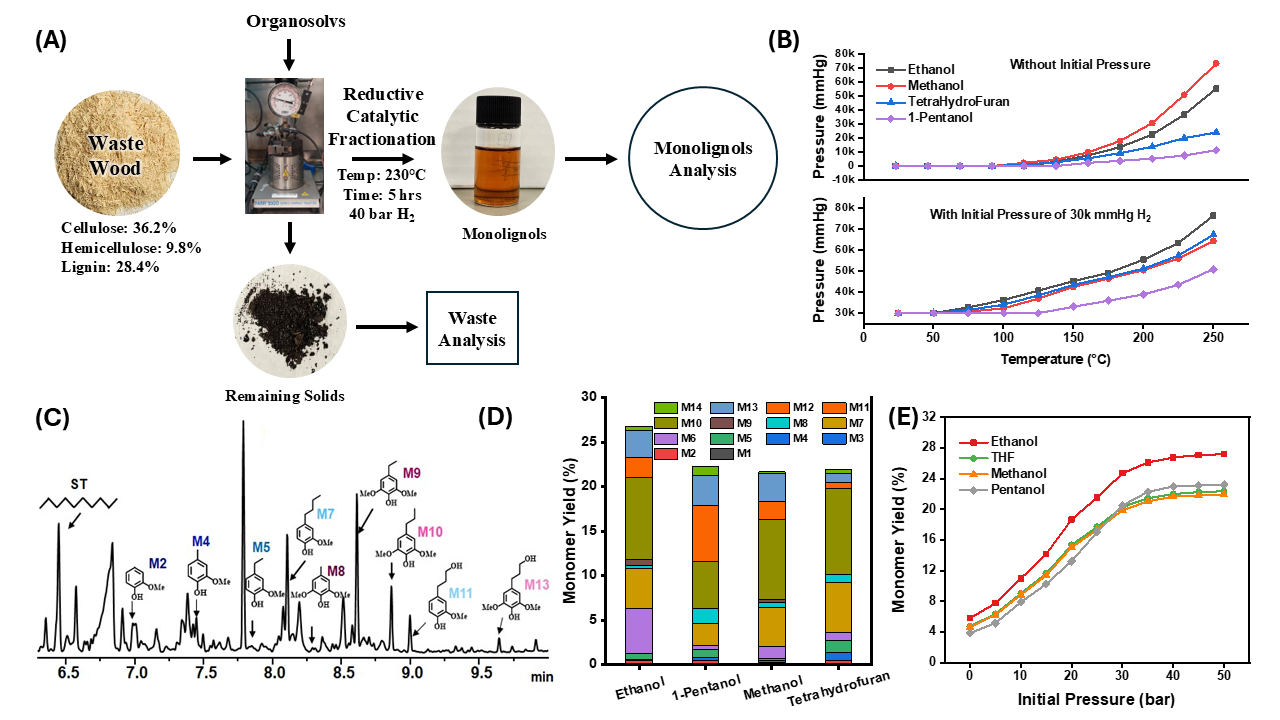
In this study, four organic solvents were evaluated for their delignification and monolignol production potential, i.e. Tetrahydrofuran (THF), Ethanol, Methanol, 1-Pentanol, and 1,4-Butanediol. Lab-scale experiments were conducted to produce monolignols (Fig.1A), and the products were analyzed using advanced techniques to select a more effective solvent system for depolymerization at lower pressures. A 500 mg sample of finely ground wood waste with a lignin content of 28.4% was loaded into a 50 mL Parr reactor along with 200 mg of Ru/C catalyst. Twenty milliliters of the desired solvent was then added to the reactor, which was tightly sealed. The reactor was purged three times and then pressurized with hydrogen gas. The reaction was carried out for 5 hours at 230°C with continuous stirring at 200 rpm. After the reaction, the contents were cooled to room temperature, and a 1 mL sample was taken for analysis using GC/MS and GC/FID, with Decane as an internal standard to quantify the monomer yield. For polar solvents, the final product was filtered and evaporated in a rotary evaporator to remove all the solvent. The remaining dry mass was dissolved in THF and analyzed using GC/MS and GC/FID.
Results and Discussion
The vapor pressure-temperature phase diagrams for different organosolvs were constructed (Fig.1B). The relationship was studied under two different scenarios: (a) no additional gas pressure, and (b) under a 40 bar H₂ pressure. As the temperature increases, the pressure requirements for all solvents rise, but the rates of increase vary significantly among the selected solvents. The introduction of an initial pressure of 40 bar/30,000 mm-Hg H₂ significantly alters the pressure profiles for all solvents. The pressure increases more gradually with temperature, indicating that the initial pressure helps stabilize the system at higher temperatures.
Ethanol and Methanol lead to much higher pressures at operating temperatures and therefore have the highest monomer yields among the solvents considered (Fig.1D&E). Methanol is more polar than ethanol and produces higher vapor pressure. However, the monolignol yield is lower due to its lower lignin solubility compared to ethanol. The reported solubility of lignin in ethanol is 87%, compared to methanol's 81%.
The vapor pressure of Methanol is higher than THF despite an insignificant difference in their boiling points because of stronger intermolecular forces, such as hydrogen bonding and dipole-dipole interactions. However, the monolignol yield is comparable, with methanol yielding 21.67% and THF yielding 21.97% monolignols, both lower than ethanol's 26.77% monomer yield. The operational vapor pressure of 1-Pentanol is much lower than that of ethanol, methanol, and THF. However, its monomer yield (22.8%) is higher than methanol and THF, making it a better alternative for use as an RCF solvent on a larger scale.
Fig.1 (A) setup of the RCF experiments; (B) phase diagrams of the studied solvents; (C) GC/FID spectra of the products with identified monolignols at various retention times; (D) monomer yields and individual monomer composition for various solvents; and (E) total monomer yields at different initial pressures
This study's findings contribute to the development of e large-scale lignocellulosic biomass into biofuel precursors. The proposed processes have the potential to improve process economics and environmental impact by lowering energy costs. The findings are relevant to researchers and industry specialists working on the commercialization of lignocellulosic biomass-based fuels. More results will be presented in the AIChE2025 conference.
References
(1) Khan, R. J.; Lau, C. Y.; Guan, J.; Lam, C. H.; Zhao, J.; Ji, Y.; Wang, H.; Xu, J.; Lee, D.-J.; Leu, S.-Y. Recent advances of lignin valorization techniques toward sustainable aromatics and potential benchmarks to fossil refinery products. Bioresource Technology 2022, 346, 126419. DOI: https://doi.org/10.1016/j.biortech.2021.126419.
(2) Dong, C.; Meng, X.; Yeung, C. S.; Tse, H.-Y.; Ragauskas, A. J.; Leu, S.-Y. Diol pretreatment to fractionate a reactive lignin in lignocellulosic biomass biorefineries. Green Chemistry 2019, 21 (10), 2788-2800. DOI: 10.1039/c9gc00596j.
(3) Khan, R. J.; Guan, J.; Lau, C. Y.; Zhuang, H.; Rehman, S.; Leu, S.-Y. Monolignol Potential and Insights into Direct Depolymerization of Fruit and Nutshell Remains for High Value Sustainable Aromatics. ChemSusChem 2024, 17 (7), e202301306. DOI: https://doi.org/10.1002/cssc.202301306.