2025 AIChE Annual Meeting
(550c) Mapping out the Global Phase Space of Intrinsically Disordered Proteins
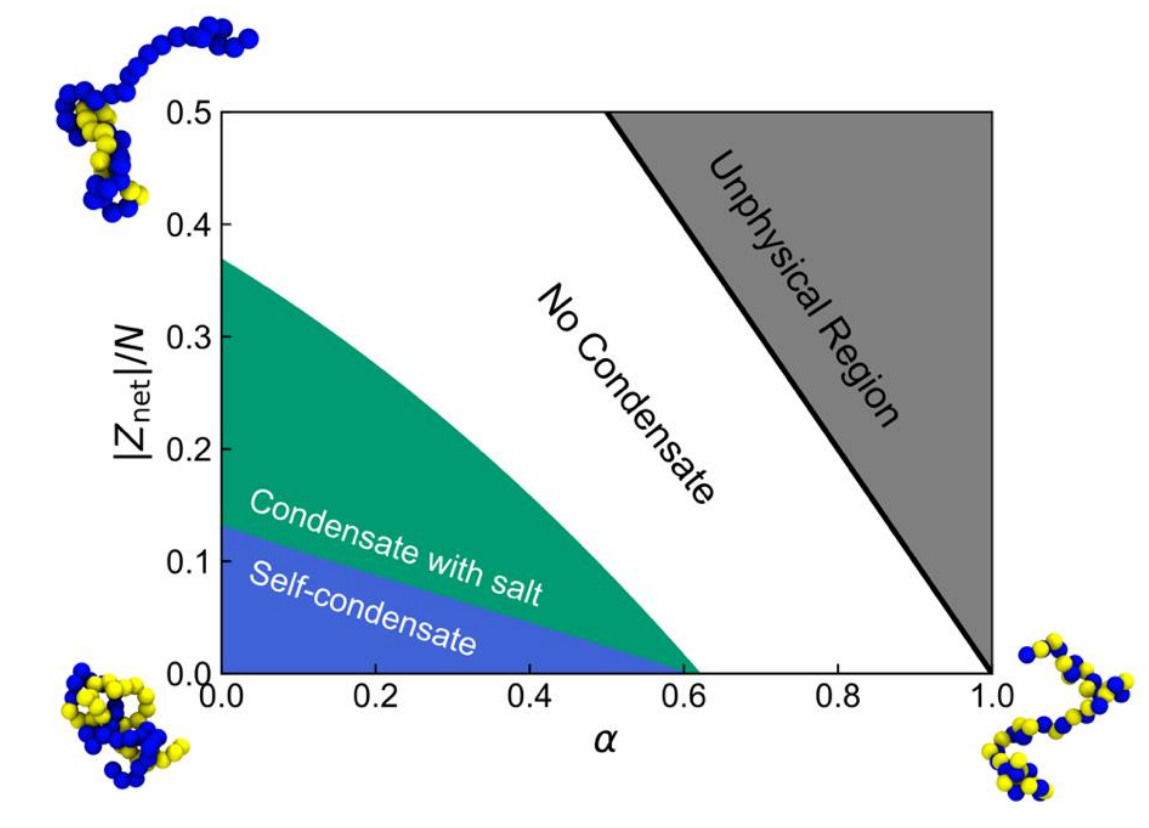
Intrinsically disordered proteins (IDPs) are capable of undergoing liquid–liquid phase
separation (LLPS), resulting in the formation of a protein-rich condensate. This phenomenon
is critical for various cellular functions and serves as a potential mechanism for drug and
gene delivery. The molecular-level protein–protein interactions are fundamental driving
forces behind LLPS, with factors such as hydrophobic, hydrogen-bonding, and electrostatic
interactions, as well as protein sequence, salt concentration, and solvent quality,
significantly influencing an IDP's ability to form condensates. To comprehensively map the
phase space of general IDPs, we employ a two-step approach. First, building upon our
successful modeling of coacervation in analogous polyelectrolyte systems, we extend our
liquid-state (LS) theory to IDPs, systematically incorporating the aforementioned factors. In
addition, we use the LS theory to study the partition of solutes (drugs, proteins, etc) between
the solution phase and the coacervate. This theoretical framework provides essential
insights, which we subsequently validate through molecular dynamics simulations. These
simulations yield additional information regarding the dynamics and structural
characteristics of the condensates. The outcomes of this research will culminate in a
detailed roadmap of the phase space of IDPs and their ability to encapsulate solute
molecules, thereby facilitating experimental navigation and accelerating the development
of application-specific IDPs
separation (LLPS), resulting in the formation of a protein-rich condensate. This phenomenon
is critical for various cellular functions and serves as a potential mechanism for drug and
gene delivery. The molecular-level protein–protein interactions are fundamental driving
forces behind LLPS, with factors such as hydrophobic, hydrogen-bonding, and electrostatic
interactions, as well as protein sequence, salt concentration, and solvent quality,
significantly influencing an IDP's ability to form condensates. To comprehensively map the
phase space of general IDPs, we employ a two-step approach. First, building upon our
successful modeling of coacervation in analogous polyelectrolyte systems, we extend our
liquid-state (LS) theory to IDPs, systematically incorporating the aforementioned factors. In
addition, we use the LS theory to study the partition of solutes (drugs, proteins, etc) between
the solution phase and the coacervate. This theoretical framework provides essential
insights, which we subsequently validate through molecular dynamics simulations. These
simulations yield additional information regarding the dynamics and structural
characteristics of the condensates. The outcomes of this research will culminate in a
detailed roadmap of the phase space of IDPs and their ability to encapsulate solute
molecules, thereby facilitating experimental navigation and accelerating the development
of application-specific IDPs

