2025 AIChE Annual Meeting
(444f) Machine Learning-Assisted Synthesis and Operation of CaO-Based Sorbents for Long Cyclic Life-Time CO2 Capture
Abstract
This study explores the machine learning-assisted design and operation of CaO-based sorbents for CO₂ capture with enhanced cyclic stability. A database comprising 1,042 reported CaO-based sorbents was compiled, containing synthesis protocols, material properties, operation condition and performance metrics. A machine learning model was trained to predict CO₂ capture capacity and decay rate, demonstrating significant improvements in accuracy. The model identified FeMn-doped CaO sorbents as promising candidates, which were experimentally validated, showing a decay rate of only 0.803% per cycle. Furthermore, the model optimized synthesis conditions, confirming 850°C as the optimal calcination temperature. This work highlights the potential of machine learning in accelerating sorbent development, optimizing synthesis protocols, and improving CO₂ capture performance.
Key words: Carbon capture, Machine learning, CaO-based sorbents
1. Introduction
Anthropogenic activities, especially the combustion of fossil fuels, emit substantial amounts of greenhouse gases into the atmosphere, contributing to global warming[1] and an increase in extreme weather events[2]. Therefore, deployment of carbon capture measures for the disposal of greenhouse gases is crucial for limiting emissions[3]. Recent main CO2 capture technologies can be categorized into pre-combustion [4], oxy-fuel combustion [5] and post-combustion techniques [6]. Their abundance, low cost and high capture capacity (0.78 gCO2 g-1CaO) of CaO-based sorbents have attracted significant commercial interest as a post-combustion technique. However, sintering-induced deactivation (~50% capacity loss) remains a major challenge[7], and hence extensive experimental efforts have been devoted to developing more stable sorbents by altering synthesis procedures and operational conditions[8]. Despite these efforts, it remains challenging to ascertain whether the "optimal" results found in experiment represent the best achievable outcomes, given the vast number of degrees of freedom in operational parameters (e.g. temperature, CO₂ partial pressure, and cycle duration) and material properties (e.g. specific surface area and porosity).
Therefore, this work extracted data from publicly published literature, compiling a tabular dataset of 1042 reported materials containing their composition, preparation protocols, material properties, operation conditions, CO2 capture capacity and stability, trained a high-precision machine learning model. This XGboost model predicted the CO2 capture capacity and decay rate of CaO-based sorbents and efficiently designed a high-performance CaO-based sorbent.
2.Methodology
2.1.Model implementation and evaluation
The XGBoost model proposed in this paper is developed based on the Gradient Boosting Decision Tree (GBDT). This XGBoost model (Figure 1) performed regression on the input and output of each stage, utilizing a separate regressor for each output label to enhance predictive accuracy while optimizing computational efficiency. The dataset (D) was partitioned into a training set (80%) and a test set (20%) without overlapping. Model performance was evaluated by comparing the predicted values with the actual values in the test set. The root mean square error (RMSE) was employed as the performance metric, calculated as equation 1,
where and denote the actual and predicted values, respectively.
2.2.Sample preparation and test
The experiment group was synthesized by sol-gel method. Initially, Ca(NO3)2.4H2O was mixed with citric acid monohydrate in solution. The specific amount of metallic nitrate and ethanol were added in the solution with sufficient mixing (30℃). Then the solution was heated to generate sol-gel. After aging 24 hours, the prepared sol-gels were dried at 120℃. After drying, the meshed powders were calcined at furnace (850℃ 2hours) and grinded to obtain the CaO-based sorbents. The molar ratio of metal ions to citric acid is 1:2, and Ca2+ to other metal ions is 9:1, which is named MCa-0c. For example, FeCa-0c is Fe3+ doping inside CaO materials.
The Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) method were implemented to analyse the characterizations. The morphologies of modified sorbents before and after CO2 sorption/desorption were observed using a scanning electron microscope, SEM (ZEISS). In the typical experiment, 1g fresh prepared CaO-based sorbents were placed in the fixed-bed reactor. The CaO-based sorbents are pretreated at 850℃ with a heating rate of 20℃/min under pure N2 flow. Then, 20% CO2 with N2 is fed into the reactor at 700℃ as carbonation stage. Pure N2 is fed into reactor at 850℃ as calcination stage. The outlet gases were analyzed by Siemens ULTRAMAT 23 to obtain CO2 concentration in pipeline.
3.Results and discussion
3.1.Database construction and preprocessing
Databases are an important foundation for machine learning to accelerate CaO-based sorbents development. This study summarized 1042 reported samples for CO2 capture from 2016 to 2023. Four principal factors form the dataset collected, namely synthesis materials and protocol, physical material properties, operation condition and reactive performance.
Many data lack the physical properties of the material, such as the microstructure, specific surface area and pore size of the adsorbent. Therefore, this study set up a two-stage machine learning process (Figure 2). The first stage simulates the relationship between the synthesis scheme, precursors, dopants and chemical treatments and the resulting material properties. The second stage examines the correlation between the input of the first stage and the material properties and the overall performance of the CaO adsorbent.
3.2.Predicted results from XGboost model
With the trained XGBoost regressors, the results show a 25% improvement in RMSE for stage 1 labels and a 40% improvement for stage 2 labels compared to the baseline (Figure 3). Firstly, the substantial reduction in RMSE indicates that the model effectively captures the complex relationships between synthesis protocols, material properties, and the performance of CaO sorbents. This demonstrates the potential of machine learning to uncover nuanced patterns in material synthesis that may not be evident through traditional methods, guiding the design of more efficient sorbents.
Moreover, the improved performance in stage 2 highlights the model's ability to connect synthesis parameters and material properties with real-world sorbent performance metrics. By accurately modeling these relationships, future research and development efforts can be guided toward more effective and economically viable sorbent materials, thereby contributing to the broader goal of optimizing carbon capture technologies.
3.3.Designed CaO-based sorbents
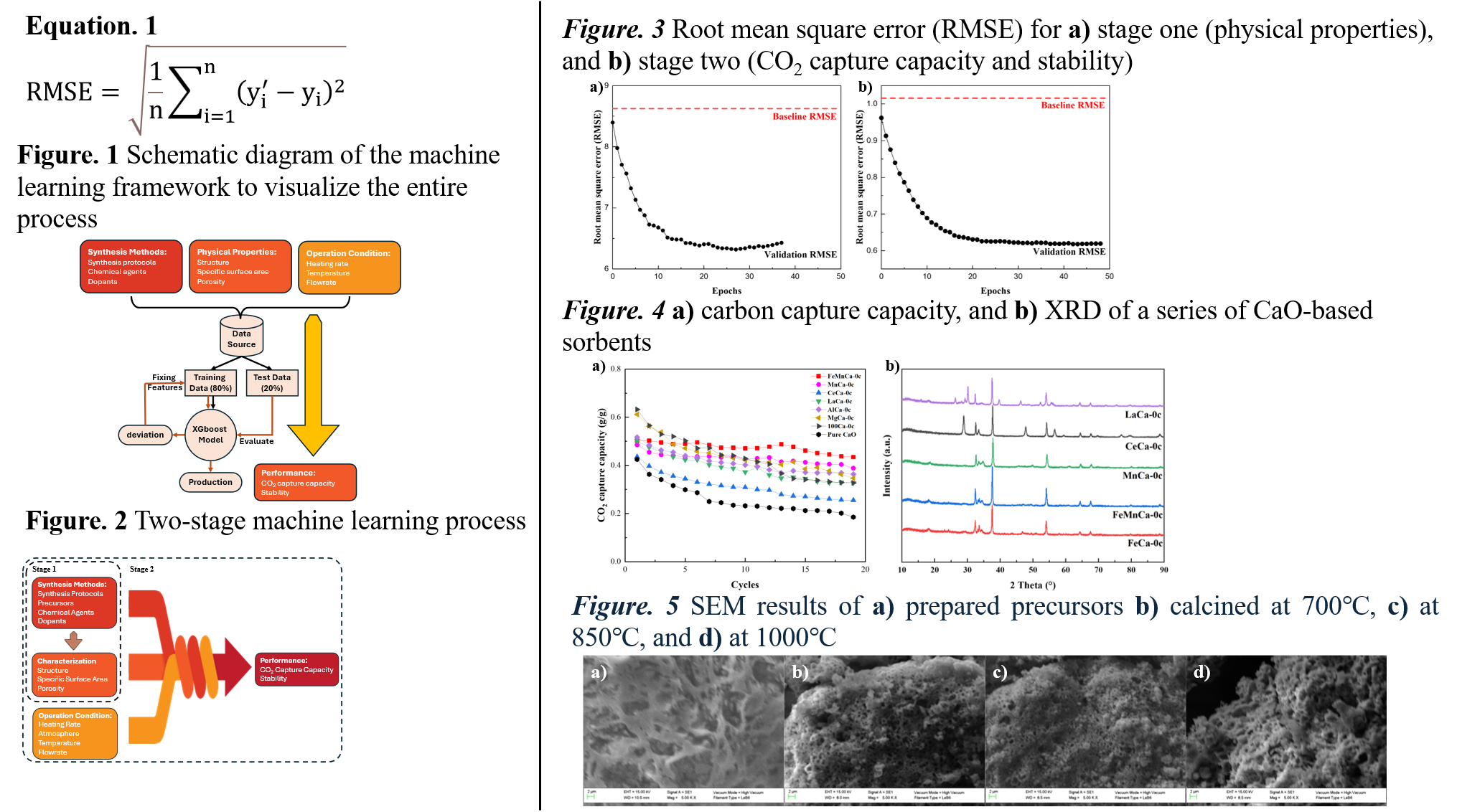
Recent popular CaO-based sorbents were doped with inert metal oxide as framework to prevent agglomerating of CaO particles during CaL process. Mg, Al, Ce and La are widely studied because of their high Tamman temperature. This work uses the above prediction model to find a FeMn-doped CaO-based sorbent would have better performance, which is different from popular studies. A series of CaO-based sorbents with various dopants were synthesized to verify these results (figure 4a). FeMn-doped material has a very high stability, with an average decay rate of only 0.803% per cycle.
Figure 5 displays the effect of calcination temperature on the synthesis protocols. After calcination, the CaO-based sorbents have sufficient porous structure. XGboost model predict that 850℃ is the optimal calcination temperature. From the experimental results, the lower calcination temperature in figure 5b has similar porous structure, compared with figure 5c (predicted temperature). However, 700℃ calcination temperature cannot sufficiently calcine the precursors. Figure 5d has worse porous structure, because CaO is obviously aggregated.
4.Conclusion
This work developed a machine learning-assisted prediction of CaO-based sorbents performance. By extracting data from the published relevant literature, a database was established. Under this machine learning framework, this work successfully obtained high-performance CaO-based sorbents. Experimental verification confirmed that the FeMn-doped CaO sorbents exhibited excellent cycling performance, while the model-guided 850°C calcination ensured optimal structural properties. These findings provide a data-driven approach to design high-performance CO₂ sorbents, accelerate material discovery and improve the efficiency of carbon capture technology.
Reference
[1] D.W. Keith. Why Capture CO2 from the Atmosphere? Science. 325 (2009) 1654-1655.
[2] C.J. Carlson, G.F. Albery, C. Merow, et al. Climate change increases cross-species viral transmission risk. Nature. 607 (2022) 555-562.
[3] A.G. Olabi, M.A. Abdelkareem. Renewable energy and climate change. Renewable and Sustainable Energy Reviews. 158 (2022) 112111.
[4] A.G. Olabi, K. Obaideen, K. Elsaid, et al. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renewable and Sustainable Energy Reviews. 153 (2022) 111710.
[5] B.J.P. Buhre, L.K. Elliott, C.D. Sheng, et al. Oxy-fuel combustion technology for coal-fired power generation. Progress in Energy and Combustion Science. 31 (2005) 283-307.
[6] W. Gao, S. Liang, R. Wang, et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges. Chemical Society Reviews. 49 (2020) 8584-8686.
[7] Z. Wang, C. Ma, A. Harrison, et al. Enhancement Strategies of Calcium Looping Technology and CaO-Based Sorbents for Carbon Capture. Small. n/a (2025) 2412463.
[8] Y. Liu, T. Zhao, W. Ju, S. Shi. Materials discovery and design using machine learning. J Materiomics. 3 (2017) 159-177.