2025 AIChE Annual Meeting
(356b) Lung-Specific Gene Delivery Via Red Blood Cell Hitchhiking Using Adeno-Associated Viruses
To address this unmet need, we employed red blood cell (RBC) hitchhiking (RH), a non-viral delivery approach designed to enhance targeted deposition of therapeutics in specific organs. RH involves adsorbing the therapeutic payload, in this case, AAV, onto the surface of RBCs, which then circulate throughout the body and release their cargo at target tissues via physical contact with the endothelium and shear-induced detachment. This platform takes advantage of the natural biomechanical interactions between RBCs and vascular endothelium, particularly in narrow capillaries where shear stress is high. Previous studies have demonstrated that RH can improve the delivery of nanoparticles and biologics to the lungs, but its application to viral vectors has remained underexplored. Here, we investigated whether RH could enhance lung-targeted AAV delivery and facilitate transduction beyond the endothelial layer following intravenous administration.
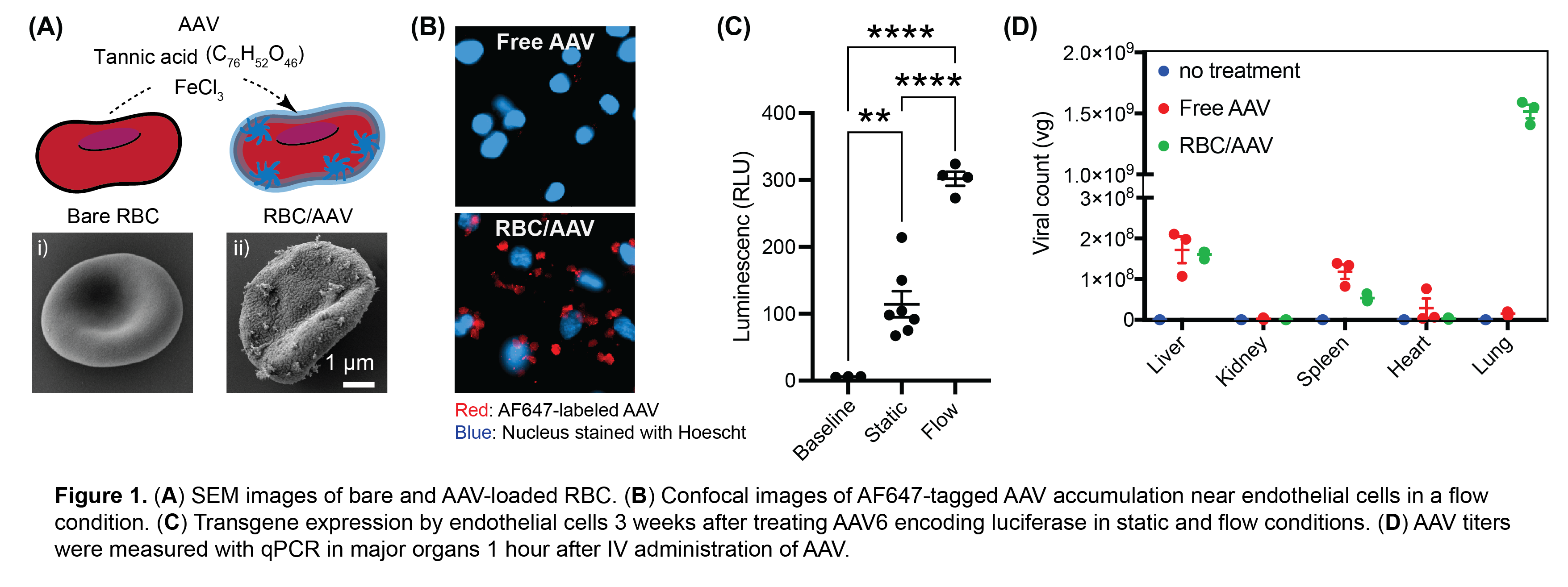
To create the RBC/AAV complex, we developed a simple, one-pot synthesis method. Murine RBCs were incubated with AAV particles in the presence of tannic acid (TA) and ferric chloride (FeCl₃), forming a supramolecular network that bound AAV to the RBC membrane. This interaction produced nanoscale clumps composed of AAV, TA, and Fe on the RBC surface, as observed by scanning electron microscopy (Figure 1A). We first tested the delivery potential of this complex under physiologically relevant conditions using a microfluidic flow chamber lined with endothelial cells. Under flow, the RBC/AAV complex showed markedly higher AAV deposition on endothelial cells compared to free AAV, highlighting the importance of RBC-endothelium contact (Figure 1B). Importantly, transduction efficiency was significantly higher when flow was applied during the treatment, indicating that shear stress promotes AAV detachment from the RBC surface and enhances uptake by target cells (Figure 1C). In vivo studies further confirmed the efficacy of RH-mediated AAV delivery. Following intravenous injection of the RBC/AAV complex in mice, we observed an approximately 100-fold increase in AAV accumulation in the lungs compared to free virus administration (Figure 1D). This accumulation translated into robust transgene expression not only within pulmonary endothelial cells but also deeper in the lung interstitium, demonstrating that RH enables trans-endothelial transport and broader tissue transduction.
These findings suggest that RBC hitchhiking significantly enhances local AAV concentration at target endothelial surfaces, allowing for efficient deposition and tissue entry. By combining physical contact-mediated delivery with shear-triggered release, RH improves AAV localization and functional transduction in target organs while reducing off-target exposure. This strategy shifts the focus from viral vector design alone to delivery optimization, offering a complementary approach to current AAV engineering efforts. Ultimately, integrating RH with rational capsid selection could unlock new possibilities for treating diseases that require efficient gene delivery to parenchymal tissues, particularly in the lung and other challenging organs.