2025 AIChE Annual Meeting
(3kh) Light-Assisted Molecular Purification (LAMP) for Recombinant Proteins Purification and Fluorogenesis
Authors
Recombinant proteins are widely used in therapeutics, vaccines, and biochemical research. Traditionally, their purification relies on column chromatography techniques such as Ni-NTA affinity or ion exchange chromatography. While effective, these methods are often limited by high costs, nonspecific binding, and complex downstream processing steps (e.g., removal of imidazole and salts), making purification a multi-step, labor-intensive process.
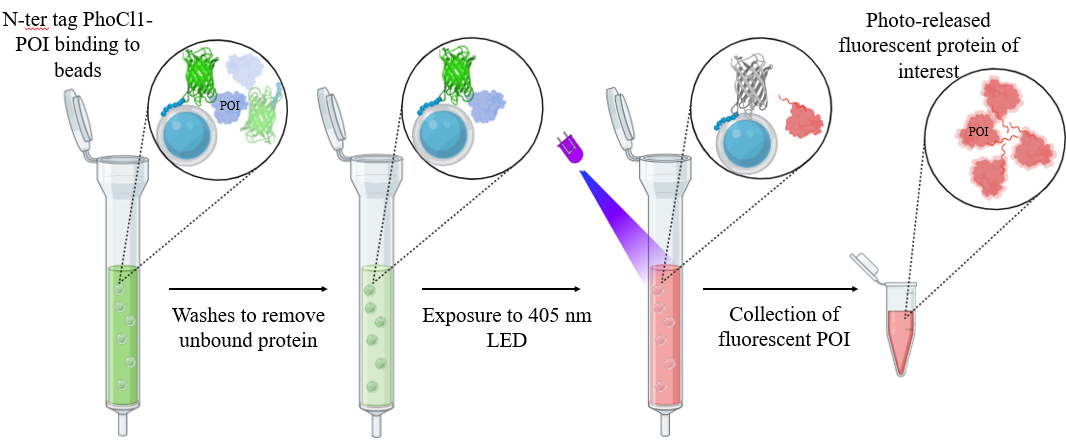
To address these challenges, we propose a novel Light-Assisted Molecular Purification (LAMP) technique. This method leverages PhoCl1, a photocleavable protein that undergoes irreversible cleavage upon exposure to 400 nm light, producing an N-terminal barrel and a C-terminal peptide fragment (CTPF). By genetically fusing the protein of interest (POI) to the C-terminus of PhoCl1—engineered with an N-terminal His-tag—the fusion protein can be immobilized on Ni-NTA resin. Upon illumination, cleavage releases the POI-CTPF into the supernatant while the His-tagged N-terminal PhoCl1 barrel remains bound to the resin.
We successfully applied this method to purify several proteins, including supercharged variants of superfolder GFP ((-30)GFP, (+15)GFP, and (+36)GFP), enzyme riboflavin kinase (MjRibK), TEV protease, a protein Maltose Binding Protein (MBP). Interestingly, MjRibK, TEV protease, and MBP purified via LAMP exhibited red fluorescence. Further investigation revealed that PhoCl1 alone was also fluorescent post-cleavage, likely due to aggregation of the CTPF fragment—a hypothesis supported by DLS, fluorescence anisotropy, and QM/MM calculations. Previous reports have suggested that PhoCl1 becomes nonfluorescent following light-induced dissociation. These findings suggest a reconsideration of the previously proposed model that PhoCl1 loses its fluorescence upon light treatment and suggest an alternative model wherein the cleaved CTPF exhibits red fluorescence due to aggregation.
Beyond purification, LAMP also offers a potential strategy for fluorescent labelling of proteins, providing both functional and analytical advantages. This technique thus presents a cost-effective, light-triggered alternative to conventional purification methods. Also, we anticipate that this approach of inducing red fluorescence in non-fluorescent biomolecules will open new possibilities for labeling and studying biomolecules in various applications.