2025 AIChE Annual Meeting
(27c) Light-Assisted Molecular Purification (LAMP) for Recombinant Protein Purification
Authors
Yashwant Kumar, IIT Gandhinagar
Manisha Ojha, IIT Gandhinagar
Karthik S. Pushpavanam, Arizona State University
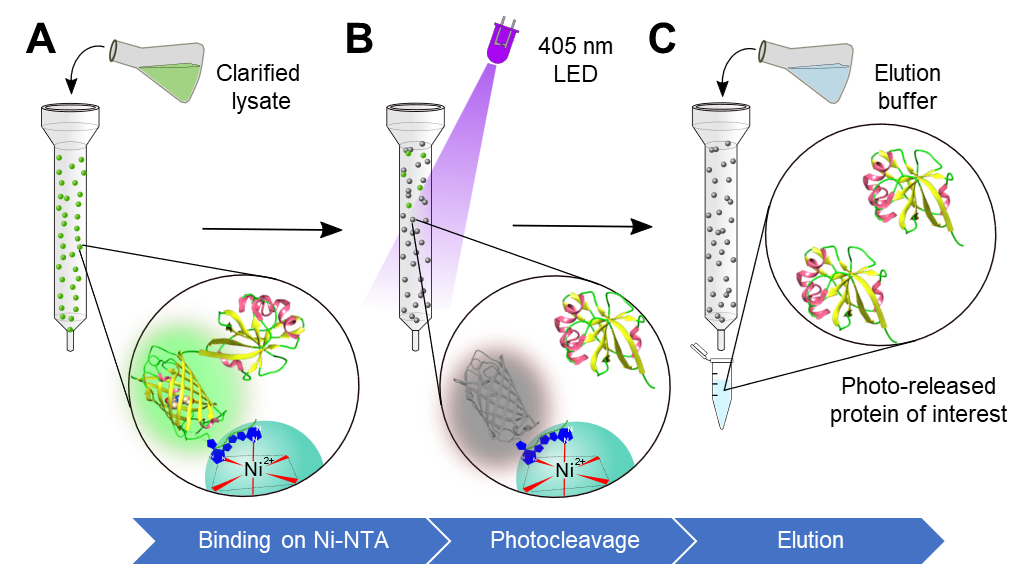
Recombinant proteins are widely used as drugs, vaccines, for therapeutics as well as in biochemical analysis. These recombinant proteins are traditionally purified through column chromatography techniques, including affinity purification using Ni-NTA or ion exchange chromatography. Despite their enormous advantages, these techniques face challenges, such as the use of expensive resins, nonspecific binding, optimization of purification processes, and tedious downstream processing steps (removal of small molecules (imidazole) and salt (NaCl) typically used in the elution buffer, and additional chromatography steps). These combined make the purification a multi-step cumbersome process. To overcome these limitations, we propose a Light Assisted Molecular Purification (LAMP) technology. We exploit a photocleavable protein, PhoCl1, which gets irreversibly cleaved upon illuminating 400 nm light, resulting in the formation of an N terminal empty PhoCl1 barrel and 9 amino acids containing C-terminal peptide fragment (CTPF). We utilize this property of the protein by genetically fusing the protein of interest (POI) to the C-terminal of the PhoCl1 protein, and the PhoCl1 protein is cloned such that it contains an N-terminal His tag. This fusion protein is then allowed to bind to the Ni-NTA resin. We demonstrate that upon illumination with 400 nm light, the PhoCl1 protein undergoes irreversible cleavage, resulting in the formation of two distinct fragments. The protein of interest, now linked to the C-terminal fragment of PhoCl1, will be released into the supernatant, whereas the N-terminal empty PhoCl1 barrel will remain bound to the Ni-NTA column. We have successfully purified several proteins using the LAMP technique, including supercharged variants of superfolder GFP (sfGFP) such as (-30)GFP, (+15)GFP, and (+36)GFP. Moreover, we have purified the enzyme riboflavin kinase (MjRibK), TEV protease and protein Maltose Binding Protein (MBP) using this technique. Additionally, we conducted experiments to investigate the impact of light on the structural integrity and functionality of the POI. The LAMP is advantageous over traditional methods as POI is eluted in the buffer of choice without the use of competitive binder molecules therefore eliminates optimization and minimizes downstream processing steps. Furthermore, there is the linear release of protein with time, providing precise control over the photorelease. We envision that this technology will offer a scalable and versatile solution for purifying sensitive proteins with diverse commercial or therapeutic applications.