2025 AIChE Annual Meeting
(485c) Life in the Balance: Energy Fluxes and Classic/Reverse Diauxie in Chronic Wound Consortia
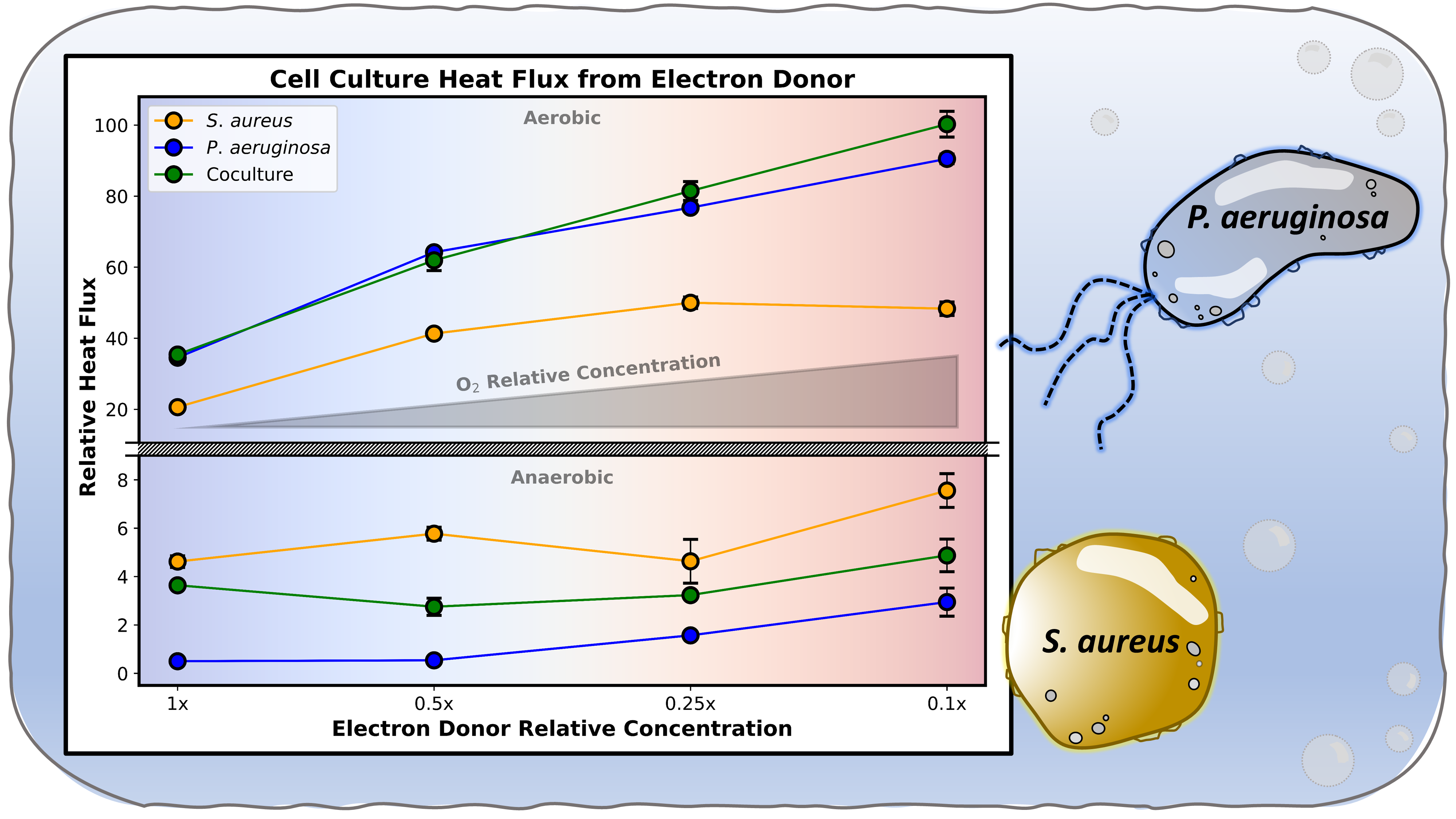
In this study, we measure consortia energy fluxes along an electron donor-to-acceptor gradient for cocultures of S. aureus and P. aeruginosa clinical isolates. The cocultures were grown in hermetically sealed batch reactors, which permitted control of the donor:acceptor ratio. Isothermal microcalorimetry analysis of the growing cultures demonstrated that the total heat accumulation varies along the donor:acceptor gradient and between monocultures and cocultures. The availability of electron acceptor was a major control parameter for coculture strain abundance, imposing over two-fold strain ratio variation across the donor:acceptor gradient. Furthermore, under oxygen-rich conditions, the coculture demonstrated the emergent property of higher conversion of electron donor into heat than either S. aureus or P. aeruginosa monocultures. Under anoxic conditions, however, the coculture produced less heat than S. aureus monocultures. Taken into consideration with biomass and metabolite flux analysis, we hypothesize that these energy flux trends depict a shift in consortia symbiosis as a function of oxygen availability. The presence of oxygen spurs competition, leading to greater stress-induced heat production. In the absence of oxygen, however, the consortium opts for a cooperative approach which lowers stress-induced heat flux and improves total community yields.