2025 AIChE Annual Meeting
(356a) Layered Lipid Nanoparticles for Ovarian Tumor-Targeted Gene Delivery
Authors
Lipid nanoparticles (LNPs) are potent transfection agents for various nucleic acid cargos. However, extrahepatic NP delivery is limited by surface opsonization, which induces aggregation and immune clearance, and off-target uptake by other cells. NP surface modification is a powerful approach to address these challenges.
The purpose of this work is to demonstrate the transfection and targeting efficacy of layered LNPs (LLNPs), or LNPs electrostatically-coated with polyanions [1]. Surface polyanion chemistry drives targeted uptake and impacts LNP biodistribution in vivo, particularly in targeting ovarian cancer tumors, greatly advantaging targeted gene delivery [2]. LLNP targeting is further applied to deliver therapeutic gene editing cargos in ovarian cancer.
Methods
To evaluate layering of LNP formulations, we encapsulated GFP-expressing mRNA or pDNA in LNPs, then electrostatically deposited carboxylated surface polyanions composed of saccharides, polypeptides or acrylic groups. LLNP transfection efficacy was assayed in vitro in EL4 lymphoma cells, RAW 264.7 macrophages, and HEK293T epithelial cells via flow cytometry. LLNP intracellular trafficking patterns were assayed via confocal microscopy studies. Intravenous LLNP biodistribution and transfection were assessed in vivo in naïve C57BL6 mice.
To evaluate ovarian cancer targeting, LLNP transfection was assayed in murine (OV2944-HM1, BPPNM) and human (OVCAR8, COV362, Kuramochi) ovarian cell lines via flow cytometry. In vivo biodistribution was assayed in a HM-1 murine model.
Results
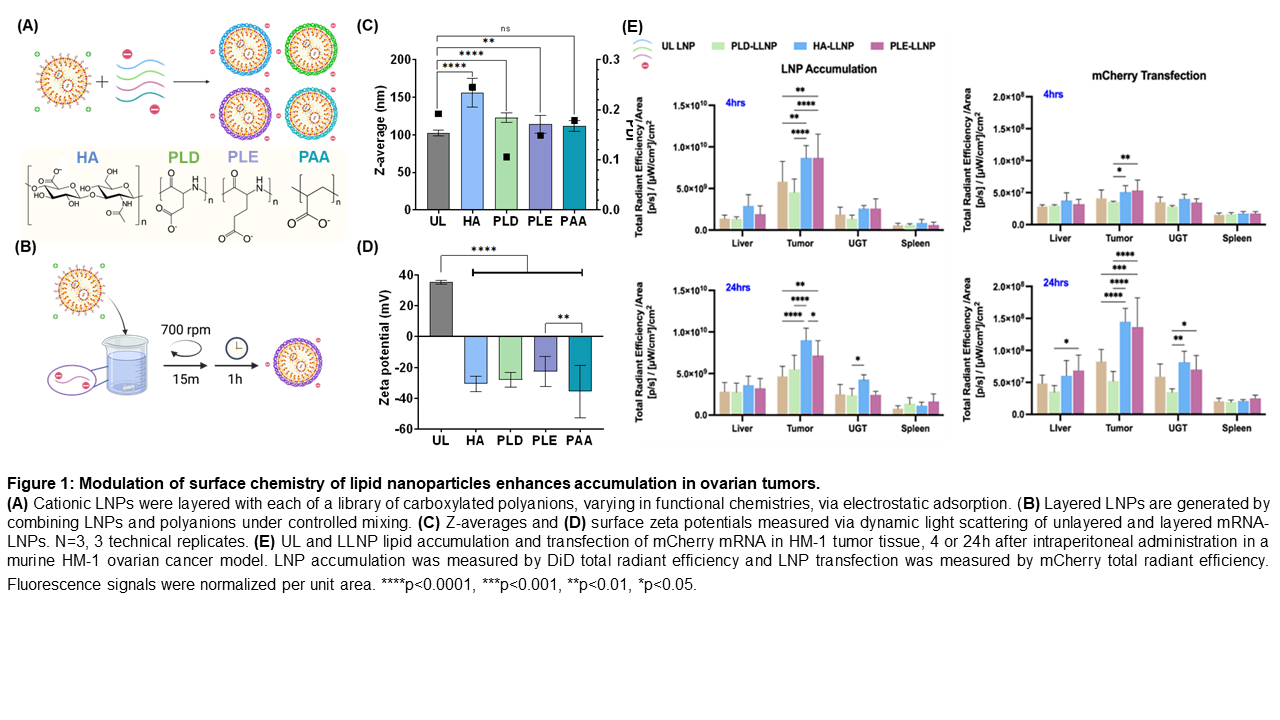
Relative to unlayered LNPs, LLNPs increased in diameter by 5 to 10 nanometers and completely reversed surface charge. mRNA and pDNA encapsulation efficiency remained consistent between unlayered and LLNPs. In in vitro screenings, LLNPs retained transfection capacity, transfecting at comparable or higher rates to unlayered LNPs. Furthermore, the choice of outer polyanion modulated transfection in a cell-dependent manner; polysaccharides, for example, improved transfection uniquely in RAW macrophages, possibly due to known CD44 expression. In vivo, polysaccharide and polypeptide outer layers improved LNP hepatic and splenic transfection; HA-layered LNPs demonstrated splenic specificity.
Surface chemistry further advantaged ovarian cancer-specific targeting; in particular, multiple polypeptide candidate outer layers improved mRNA transfection two-fold in mouse and human ovarian cancer cells, as well as in a murine ovarian tumor model.
Conclusions:
This work demonstrates that electrostatic surface modification of LNPs is a generalizable and tunable approach to achieving targeted transfection, which remains a major goal within the field of gene delivery. In particular, polypeptide-coated LLNPs significantly boost transfection in ovarian cancer lines in vitro and tumored tissue in vivo. Just as key surface chemistries drive ovarian tumor targeting, rationally chosen outer layers offer high potential for targeted gene delivery within other diseases.
Acknowledgements: We acknowledge funding from the Bill and Melinda Gates Foundation (grant INV-050202), National Science Foundation (Grant No. 2141064), and Koch Institute’s Marble Center for Cancer Nanomedicine.
References
[1] Nabar et al. Proceedings of the National Academy of Sciences 121(11). 2024.