2025 AIChE Annual Meeting
(538c) Kinetic Modeling of Electrocoagulation in Moderate to Slightly Acidic Media for Water Treatment
Authors
Akshat Verma, Texas A&M University
Shankar Chellam, Texas A&M University
Benjamin Wilhite, Texas A & M University
Jodie Lutkenhaus, TAMU
Water security is a grand challenge facing society today. With an increase in global
industrialization, more bodies of water are at risk of contamination by industrial
effluents. One method of decontamination of water is electrocoagulation.
Electrocoagulation uses electrical currents to remove contaminants such as heavy
metals, phosphates, suspended solids, organic matter and even pathogens from
wastewater. Understanding the chemical kinetics of active species including radicals
and peroxides in this process is essential for a more in-depth understanding of the
impurity removal.
The aim of this project is to develop and validate a kinetic model for an
electrocoagulation process in moderate to slightly acidic media for water treatment,
guided by experimental data. The system consists of iron electrodes (or iron and
carbon electrodes) and simulated wastewater at pH values of 5.0,5.5, 6.0 and 6.5 to
which a constant current is applied. We consider relevant electrochemical surface
reactions taking place at each respective electrode as well as homogenous reactions
(i.e., Fenton reaction) taking place in the bulk electrolyte. Using non-linear regression,
our model estimates the best-fit values for kinetic parameters such as the exchange-
current density (io), overpotential (ηs ), the volumetric mass transfer coefficient for oxygen
mass transfer (kLa) and rate constants for the Fenton reaction and oxidation of ferrous
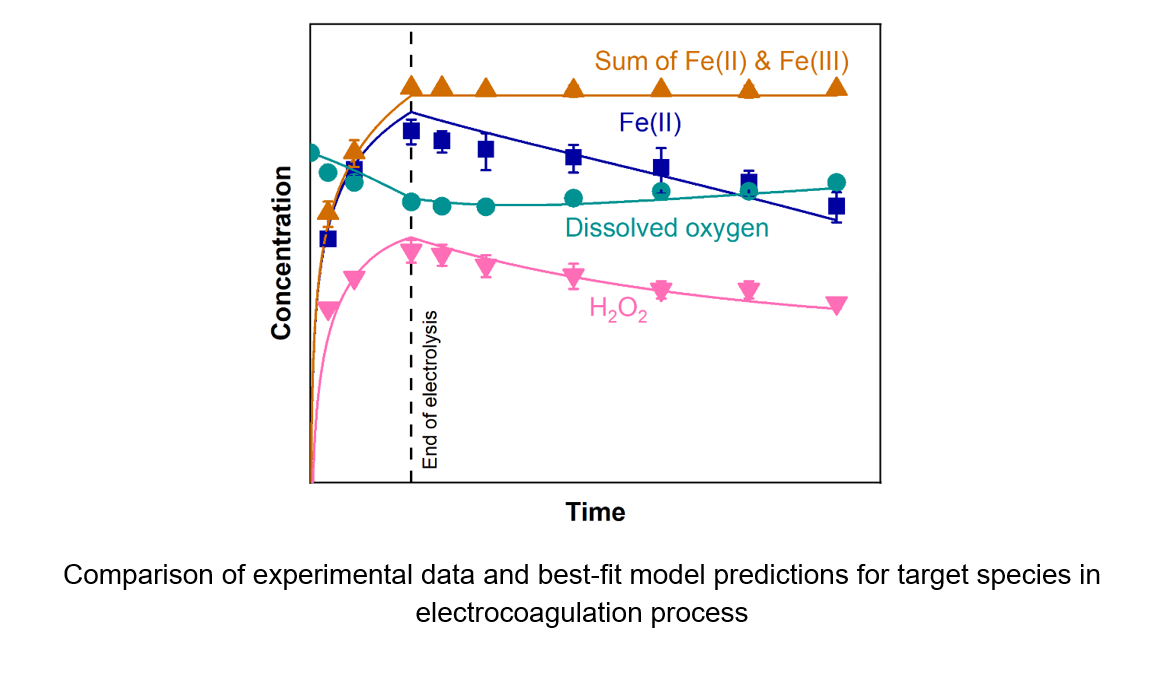
iron ions (Fe(II)) under the experimental conditions provided. The model performance is
assessed by calculating the error between model predictions and experimental data
provided for target species concentrations: Fe(II), Fe(III), dissolved oxygen (D.O), and
hydrogen peroxide (H2O2 ). The kinetic model predicts the concentrations of the target
species over time with high accuracy while providing estimates for electrochemical and
homogeneous kinetic parameters across the different pH levels. Results indicate
slower electrochemical kinetics with an increase in pH while homogenous rate
constants increased with higher pH levels. The impact of this work is that
electrochemical models of electrocoagulation can be utilized for scale-up design and
selection of reaction conditions.
industrialization, more bodies of water are at risk of contamination by industrial
effluents. One method of decontamination of water is electrocoagulation.
Electrocoagulation uses electrical currents to remove contaminants such as heavy
metals, phosphates, suspended solids, organic matter and even pathogens from
wastewater. Understanding the chemical kinetics of active species including radicals
and peroxides in this process is essential for a more in-depth understanding of the
impurity removal.
The aim of this project is to develop and validate a kinetic model for an
electrocoagulation process in moderate to slightly acidic media for water treatment,
guided by experimental data. The system consists of iron electrodes (or iron and
carbon electrodes) and simulated wastewater at pH values of 5.0,5.5, 6.0 and 6.5 to
which a constant current is applied. We consider relevant electrochemical surface
reactions taking place at each respective electrode as well as homogenous reactions
(i.e., Fenton reaction) taking place in the bulk electrolyte. Using non-linear regression,
our model estimates the best-fit values for kinetic parameters such as the exchange-
current density (io), overpotential (ηs ), the volumetric mass transfer coefficient for oxygen
mass transfer (kLa) and rate constants for the Fenton reaction and oxidation of ferrous
iron ions (Fe(II)) under the experimental conditions provided. The model performance is
assessed by calculating the error between model predictions and experimental data
provided for target species concentrations: Fe(II), Fe(III), dissolved oxygen (D.O), and
hydrogen peroxide (H2O2 ). The kinetic model predicts the concentrations of the target
species over time with high accuracy while providing estimates for electrochemical and
homogeneous kinetic parameters across the different pH levels. Results indicate
slower electrochemical kinetics with an increase in pH while homogenous rate
constants increased with higher pH levels. The impact of this work is that
electrochemical models of electrocoagulation can be utilized for scale-up design and
selection of reaction conditions.

