2025 AIChE Annual Meeting
(319g) The Kinetic Consequences of Water on Catalytic Methane Decomposition for Hydrogen and Carbon Nanotube Production
Authors
Phuong Nguyen Thi - Presenter, University of Oklahoma
Laura Gomez Gomez, university of Oklahoma
Caleb Bavlnka, University of Oklahoma
Le Thy Thy Ho, University of Oklahoma
Bin Wang, The University of Oklahoma
Daniel E. Resasco, University of Oklahoma
Steven P. Crossley, University of Oklahoma
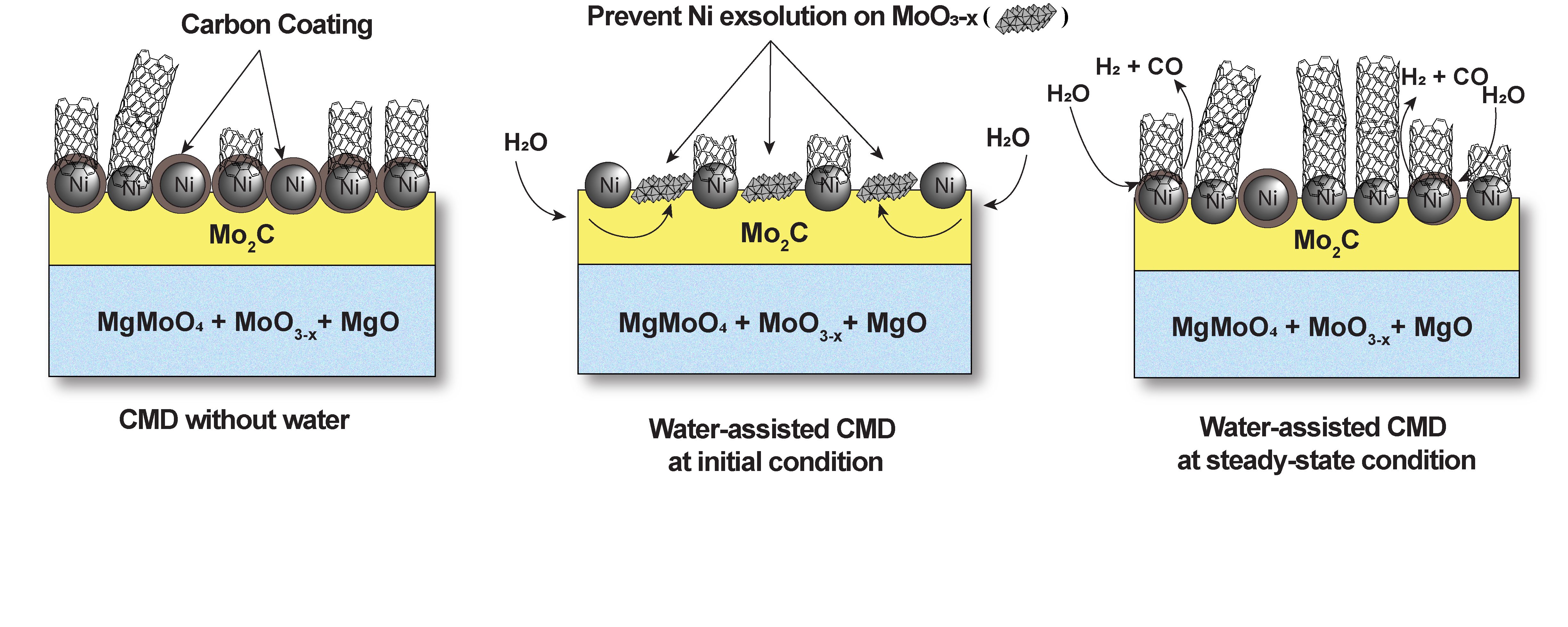
The growing demand for low-CO₂ alternative energy sources is driving research toward renewable solutions, with hydrogen production from biomass and natural gas emerging as a key focus. In this study, we investigate the impact of water, which is present in and generated from biomass-derived streams, on carbon nanotube (CNT) growth and hydrogen production during methane decomposition over Ni-Mo/MgO. However, we reveal here that the impact of water on CNT growth depends on the stage at which the water is incorporated. When water is introduced at the beginning of methane decomposition, methane conversion rates are negatively impacted, as the Ni-Mo/MgO catalyst undergoes significant changes during the carburization stage [1]. In contrast, the incorporation of 3% water after reaction stabilization enhances the rate of hydrogen production by as much as 19% at 800ºC. We hypothesize that water selectively reacts with the amorphous carbon that leads to catalyst deactivation, thus enabling further activity of the most active sites. Further study of water incorporation at steady state across different temperatures reveals that hydrogen production rates are significantly enhanced by water at lower reaction temperatures. Moreover, steady-state water incorporation reduces the apparent activation energy from 240±11 kJ/mol without water to 58±14 kJ/mol with 16 Torr of water. DFT calculations reveal that water preferentially interacts with carbon fragments on the catalyst surface to remove carbon deposits with a barrier lower than that required for methane activation, further supporting its role in cleaning active sites on the catalyst surface. The characterization of the formed carbons reveals the formation of more graphitic materials produced in the presence of water, further supporting the impact of water on the properties of nanotubes. These results provide clarity toward the many ways in which water, or co-feeding of biomass-derived materials, may impact catalytic methane pyrolysis rates.
[1]Gomez et al.,Cell Reports Physical Science(2025),10.1016/j.xcrp.2025.102519