2025 AIChE Annual Meeting
(482a) Kinetic Analysis - Driven Strategies for Enhancing Glycolate Production from Formaldehyde in Fed-Batch Bioreactors
The increasing concentration of carbon dioxide (CO₂) in the atmosphere is a critical environmental challenge, which requires mitigation strategies such as carbon capture and utilization (CCU)1. Converting CO₂ into soluble one-carbon (C1) compounds like formate and formaldehyde presents an opportunity for biomanufacturing valuable chemicals2. Advances in synthetic biology and metabolic engineering have enabled efficient C1 bioconversion into longer-chain carbon molecules using engineered microorganisms3. One such approach involves the production of glycolate from formaldehyde using engineered Escherichia coli strains.
Previous research developed a strain that successfully converts formaldehyde into glycolate at a relevant titer4, through the FORCE pathways5, which consists of the ligation of formyl-CoA with carbonyl-containing molecules catalyzed by the enzyme HACS6. The experiments were conducted only at a small scale (96-well microplates, 1 mL), where the optimized conditions included M9-LB media with 20 g/L glycerol, 30 °C, 1000 rpm, a 30-minute batch period, and an initial substrate concentration of 5 mM. To improve process efficiency and scalability, process engineering tools and kinetic modeling were employed. Bioprocess scale-up faces challenges such as variations in mixing, oxygen transfer, and shear stress, which can impact cell growth and product yield7. Kinetic modeling provides valuable insights into reaction dynamics, aiding in efficient bioprocess scale-up8.
Preliminary experiments demonstrated the successful scale-up of the bioconversion process in two stages: transitioning from microplates (1 mL) to flasks (50 mL working volume) and then to bioreactors (300 mL working volume) using a fed-batch strategy to avoid substrate accumulation in the system due to its toxicity at a higher concentration. Monitoring dissolved oxygen revealed that substrate addition caused a sharp drop, followed by rapid recovery, indicating fast reaction kinetics. This study aimed to investigate in vivo bioconversion kinetics and develop strategies to enhance glycolate production in a fed-batch bioreactor, optimizing overall bioprocess performance.
Methodology
The host strain used was an engineered E. coli MG1655 derivative, carrying gene knockouts for formaldehyde oxidation (∆frmA) and formate oxidation (∆fdhF, ∆fdnG, ∆fdoG), as well as glycolate utilization (∆glcD). The strain was transformed with two plasmids (pCDF-P1-CfhHACS and pET-pCT5-LmACR-StEutE-AldA), enabling overexpression of enzymes essential for glycolate synthesis.
The bioprocess consisted of two phases: Growth and Protein Expression (GPE) and Bioconversion (BCN). In the GPE phase, cells were grown in M9-LB media with 20 g/L glycerol. After 2.5 hours, IPTG and Cumate were added to induce enzyme expression. After 24 hours, cells were harvested and washed to remove residual carbon sources. In the bioconversion (BCN) phase, cells were resuspended in M9 media, and formaldehyde (FALD) was added to initiate bioconversion. Samples were collected and analyzed for glycolate, formate (by-product), and remaining substrate.
For kinetic experiments, bioconversion was performed in 250 mL flasks (50 mL working volume) at 30ºC and 300 rpm. Samples were collected at defined intervals, and reactions were halted using 10% sulfuric acid. Supernatants were analyzed via HPLC (Shimadzu, 2021) at a 42ºC and 0.3 mL/min for glycolate and formate concentrations, while formaldehyde was quantified using the Nash protocol9. Experiments were conducted at four initial formaldehyde concentrations (5, 4.4, 3, and 2 mM) to assess substrate effects on reaction kinetics.
To enhance glycolate production, two fed-batch strategies were tested in bioreactors: (1) reducing the interval between substrate additions and (2) continuous substrate feeding. Intermittent fed-batch experiments were conducted with 30- and 20-minute intervals, while continuous feeding tested four formaldehyde flow rates (0.19, 0.14, 0.08, and 0.04 mmol/min). These strategies aimed to balance substrate addition and consumption, maintaining high bioconversion rates while preventing toxic formaldehyde accumulation.
Results and Discussion
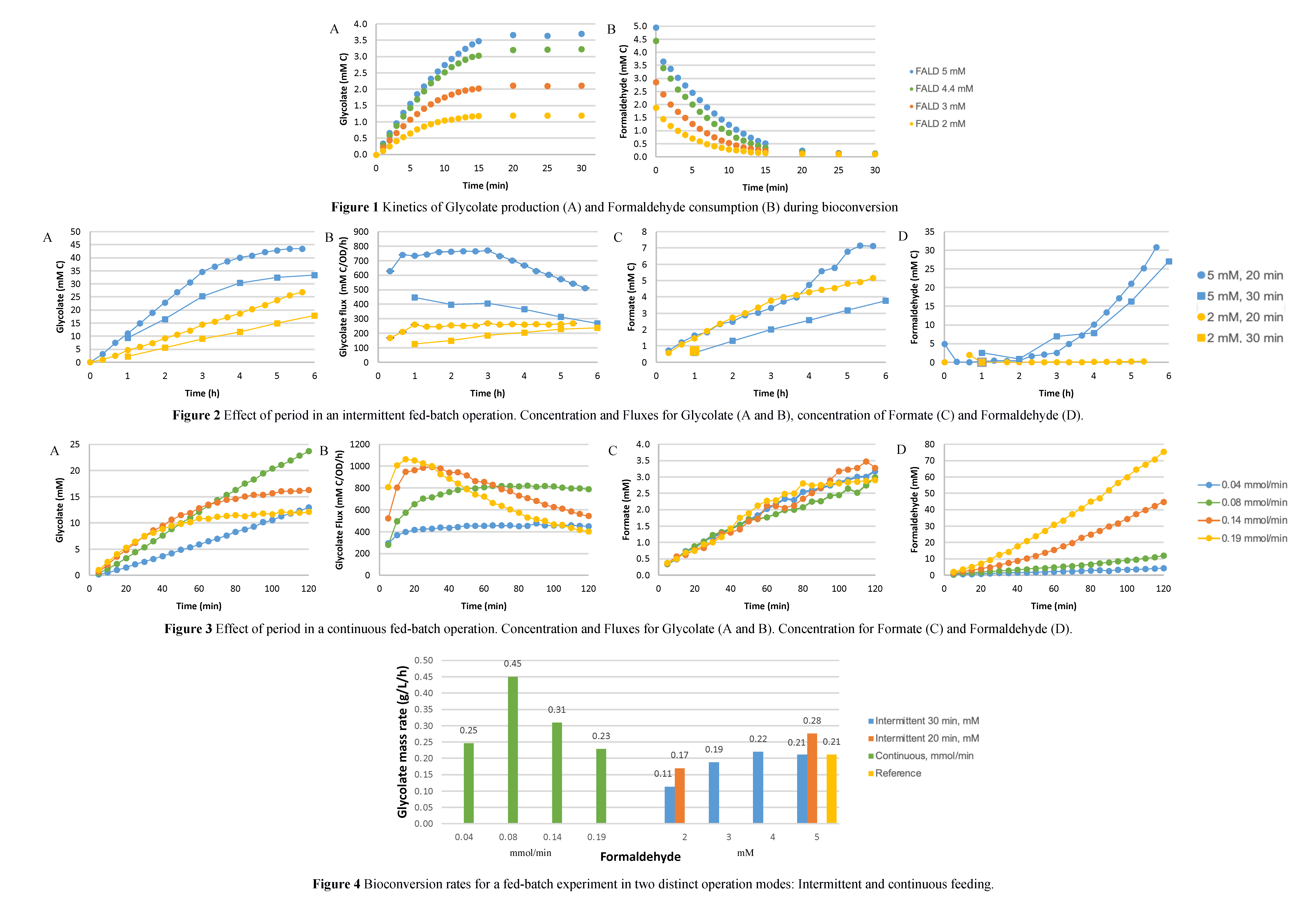
The kinetics experiments were conducted. The bioconversion process reached completion in approximately 15 minutes, achieving the highest glycolate concentration, irrespective of the initial FALD concentration, as can be seen in Figure 1. The plateau values showed a linear trend across different concentrations, indicating the glycolate concentration in the plateau as a function of the initial FALD concentration within the tested range of 2 to 5 mM. Those findings from the kinetic experiments are significant because it effectively reduced the bioconversion time from 30 to 15 minutes compared to previously published data. This reduction in the bioconversion period, in the macroscale means the reduction of the bioreactor volumes, increasing the process efficiency and feasibility.
The reaction rates are initially very high but decrease rapidly over time, and they are dependent on the substrate concentration, which also declines quickly. The key strategy to improve glycolate titer is to sustain higher and more stable reaction rates for an extended period, preventing a sharp decline to baseline levels. This can be achieved by increasing the substrate concentration while ensuring it remains within a non-toxic range. Two feeding strategies were implemented to improve glycolate titer by optimizing reaction rates. The first approach involved reducing the interval between batches. Although the initial substrate concentration remained the same for each batch, shortening the time between additions helped maintain higher reaction rates, preventing them from dropping to baseline levels. This strategy kept the cells more metabolically active, ultimately enhancing bioconversion efficiency. The second strategy involved the continuous addition of substrate. This approach aimed to find the highest feasible molar flow rate that equalizes substrate feeding and consumption, preventing substrate accumulation in the system while sustaining optimal reaction conditions.
In the first strategy, bioreactor experiments were conducted by reducing the interval between substrate additions from 30 to 20 minutes. Two initial substrate concentrations, 2 mM and 5 mM, were tested. In both cases, glycolate production increased when the substrate was supplied at shorter time intervals. For the 20-minute bioconversion, glycolate concentrations reached 26.8 mM C (50% increase) and 43.5 mM C (30% increase) for initial concentrations of 2 mM and 5 mM, respectively. Glycolate concentrations are expressed in a carbon base. In comparison, the 30-minute interval resulted in lower glycolate concentrations of 17.91 mM C and 33.38 mM C for the same initial substrate levels. Notably, for the 2 mM condition, the reaction rate remained constant throughout the process, indicating that the increased number of batches did not negatively affect cell viability. However, formate production was higher in the 20-minute bioconversion due to the greater number of batches and the higher total substrate input over the same duration. No FALD accumulation was observed at the 2 mM concentration, while at 5 mM, both experimental curves followed a similar profile. These results are illustrated in Figure 2.
In the second strategy, bioconversion was conducted in continuous feeding mode, testing four FALD flow rates: 0.19, 0.14, 0.08, and 0.04 mmol/min. The goal was to identify the highest flow rate that balances feeding and consumption. Results showed stable glycolate production at 0.04-0.08 mmol/min with minimal FALD accumulation, while higher rates (0.14 and 0.19 mmol/min) led to substrate accumulation early in the process. Compared to 30-minute fed-batch experiments, glycolate production increased by 40%. Formate production remained independent of substrate concentration. Those results can be observed in Figure 3. The bioconversion lasted only 2 hours, constrained by reactor volume and pump limitations.
In summary, both fed-batch operations could improve the glycolate production, as it shown in Figure 4. The continuous operation was the most promising strategy, allowing the duplication of the rate compared to the reference. Further improvements can be achieved by fine-tuning flow rates within 0.04–0.08 mmol/min and incorporating external micropumps.
Conclusion
Kinetic experiments provided crucial insights into reaction dynamics, product formation, and substrate consumption, highlighting the necessity of fed-batch operation to mitigate substrate toxicity. Both intermittent and continuous fed-batch strategies improved glycolate titer and production rate, with continuous formaldehyde addition demonstrating the best performance. Future research will focus on refining the optimal molar flow rates (0.04–0.08 mmol/min) and extending bioconversion duration to further enhance glycolate production efficiency in the bioreactor.
References
- Urban, M. C. Climate change extinctions. Science 386, 1123–1128 (2024).
- Naims, H. Economics of carbon dioxide capture and utilization—a supply and demand perspective. Environ Sci Pollut Res 23, 22226–22241 (2016).
- Orsi, E., Nikel, P. I., Nielsen, L. K. & Donati, S. Synergistic investigation of natural and synthetic C1-trophic microorganisms to foster a circular carbon economy. Nat Commun 14, 6673 (2023).
- Lee, S. H. et al. Identification of 2-Hydroxyacyl-CoA Synthases with High Acyloin Condensation Activity for Orthogonal One-Carbon Bioconversion. ACS Catal. 13, 12007–12020 (2023).
- Chou, A., Lee, S. H., Zhu, F., Clomburg, J. M. & Gonzalez, R. An orthogonal metabolic framework for one-carbon utilization. Nat Metab 3, 1385–1399 (2021).
- Chou, A., Clomburg, J. M., Qian, S. & Gonzalez, R. 2-Hydroxyacyl-CoA lyase catalyzes acyloin condensation for one-carbon bioconversion. Nat Chem Biol 15, 900–906 (2019).
- Shuler, M. L. & Kargi, F. Bioprocess Engineering: Basic Concepts. (Prentice Hall PTR, Upper Saddle River, NJ, 2008).
- Yang, M., Ma, Z., Chen, L., Sun, W. & Zhao, L. Process Optimization and Kinetic Modeling for Sucrose Acylation to Sucrose-6-acetate through the Dibutyltin Oxide Method. Ind. Eng. Chem. Res. 63, 9371–9379 (2024).
- Nash, T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochemical Journal55, 416–421 (1953).