2025 AIChE Annual Meeting
(254d) Isopotential Electron Titration for Quantifying Metal-Adsorbate Charge Transfer
Authors
The extent of charge transfer was measured by modifying bulk-phase hydrogen activity while forcing a metal-insulator-semiconductor (MIS) stack to electrochemical equilibrium. The system alternated between 0.5% and 100% hydrogen streams, where hydrogen adsorption/desorption generated a charge, which titrated through an external circuit.
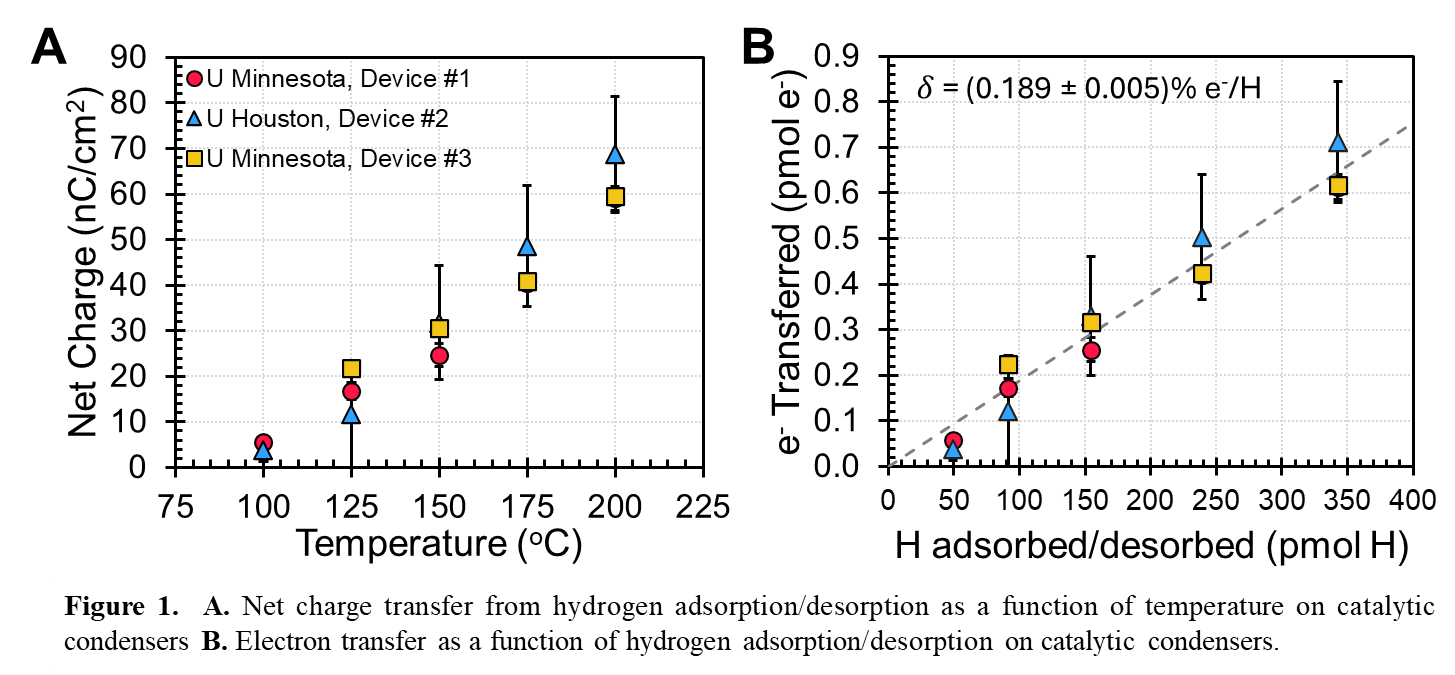
Results showed hydrogen adsorption induced a net charge transfer to the platinum surface, scaling linearly with temperature (Figure 1A). Normalized per hydrogen atom, each donated 1.89 ± 0.005 mm e⁻ mol Pt⁻¹ (Figure 1B), consistent with a Bader charge analysis, which predicted 4 mm e⁻ mol Pt⁻¹. Upon desorption, an equal and opposite charge transfer occurred. The relationship between charge transfer and work function changes was linear for small coverage changes and exponential for larger ones due to Fermi level shifts. Understanding these interactions help predict shifts in kinetic regimes, potentially changing the rate-determining step. This provides catalysts an opportunity to surpass the Sabatier optimum and achieve enhanced reaction rates.
This study introduces an electronic approach to analyzing catalyst surfaces, demonstrating how metal-adsorbate charge transfer influences catalytic properties. The methodology extends beyond hydrogen to key molecules such as carbon monoxide, oxygen, and ammonia, supporting improved catalyst design and optimization strategies.