2025 AIChE Annual Meeting
(462h) Isopotential Electron Titration: Quantifying Hydrogen Metal-Adsorbate Charge Transfer
Authors
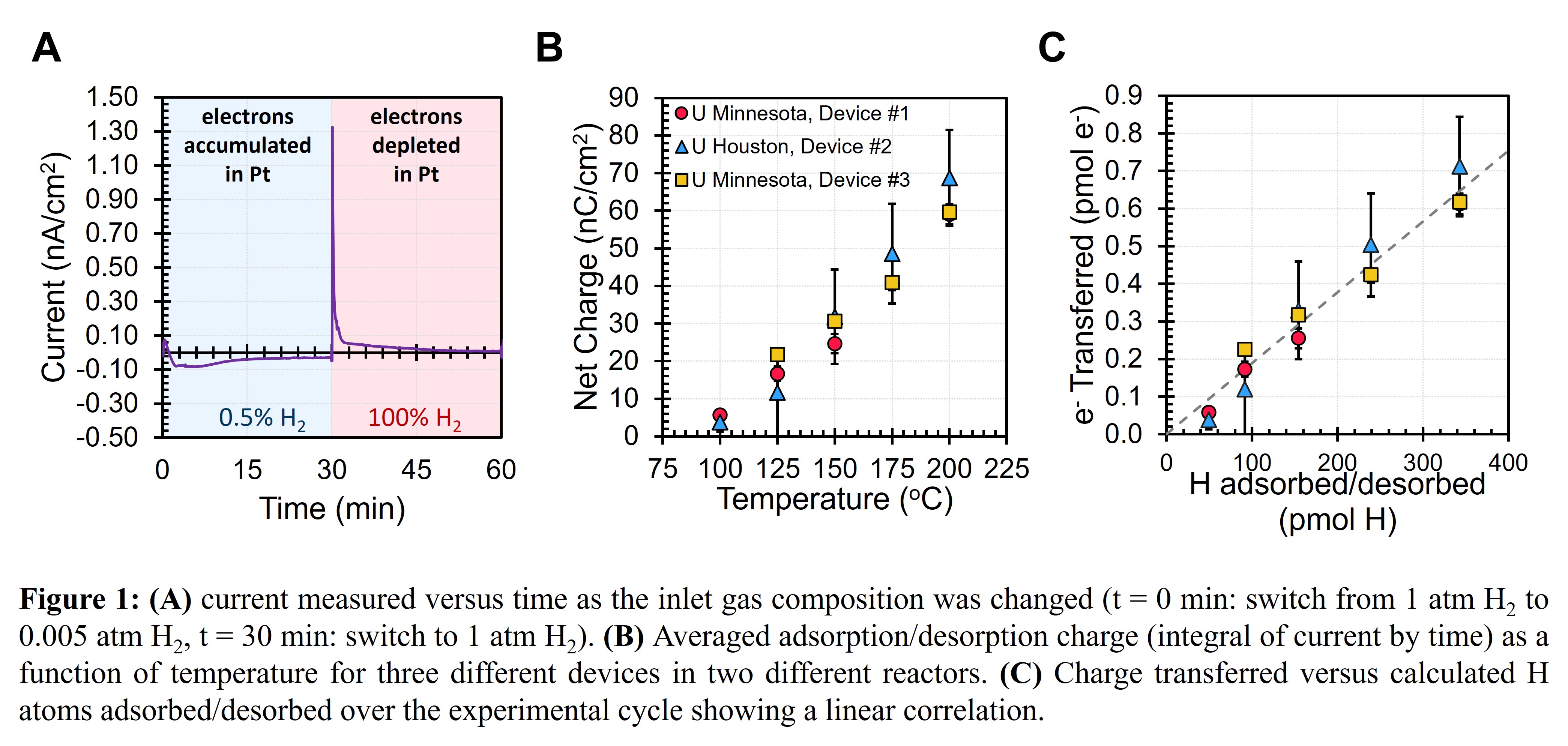
Figure 1A shows the current versus time while the composition was cycled. Switching to lower partial pressures resulted in a broad negative current peak while switching to higher partial pressure resulted in a narrow positive current peak. The integrations of these currents (i.e., charge) were equal and opposite in value and monotonically increased from 100 to 200 oC as shown in Figure 1B. Accounting for the change in coverages at each temperature using literature data [3], the amount of H* adsorbed/desorbed over the cycles was calculated. Figure 1C shows that the charge measured versus H* adsorbed/desorbed yielded a linear correlation, suggesting that each H* adsorption event donated 0.19% of an electron to the Pt surface. This extent of charge transfer allows us to calculate the change in binding energy for a given catalyst surface voltage drop. The isopotential titration technique can be used to measure the extent of charge transfer for a range adsorbates and catalysts allowing researchers to predict the effects of dynamic voltages on catalyst surface coverages.
[1] Onn., T.M., et al. J. Am. Chem. Soc. 144, 48 (2022)
[2] Hopkins, J., Page, B., et al. ChemRxiv (2025)
[3] García-Diéguez, M., et al. J. Phys. Chem. C 123, 12 (2019)