2025 AIChE Annual Meeting
(602e) Ionomer Adsorption to Functionalized Carbon Particles in Fuel Cell Catalyst Inks
Authors
A strategy used to improve ionomer distribution in the catalyst layer is to functionalize/dope carbon support particles to promote favorable interactions between the ionomer and carbon support.3,4 Such carbon functionalizations, particularly with N containing groups, have been demonstrated to significantly improve H+ and O2 transport in the catalyst layer. 3,4
We interrogate the origin of improved ionomer uniformity on functionalized carbons by characterizing interactions between ionomer and functionalized carbon support particles within the precursor ink from which the catalyst layer is deposited. More specifically, we quantify the amount of ionomer that adsorbs to functionalized carbon particles within the ink. To do so, carbon particles with adsorbed ionomer are sedimented from the ink via centrifugation, and the ionomer content of the sediment is characterized with thermogravimetric analysis. The ionomer adsorption is characterized for carbons with different N containing or SO3 functional groups which were previously shown to significantly affect fuel cell performance. 3,4
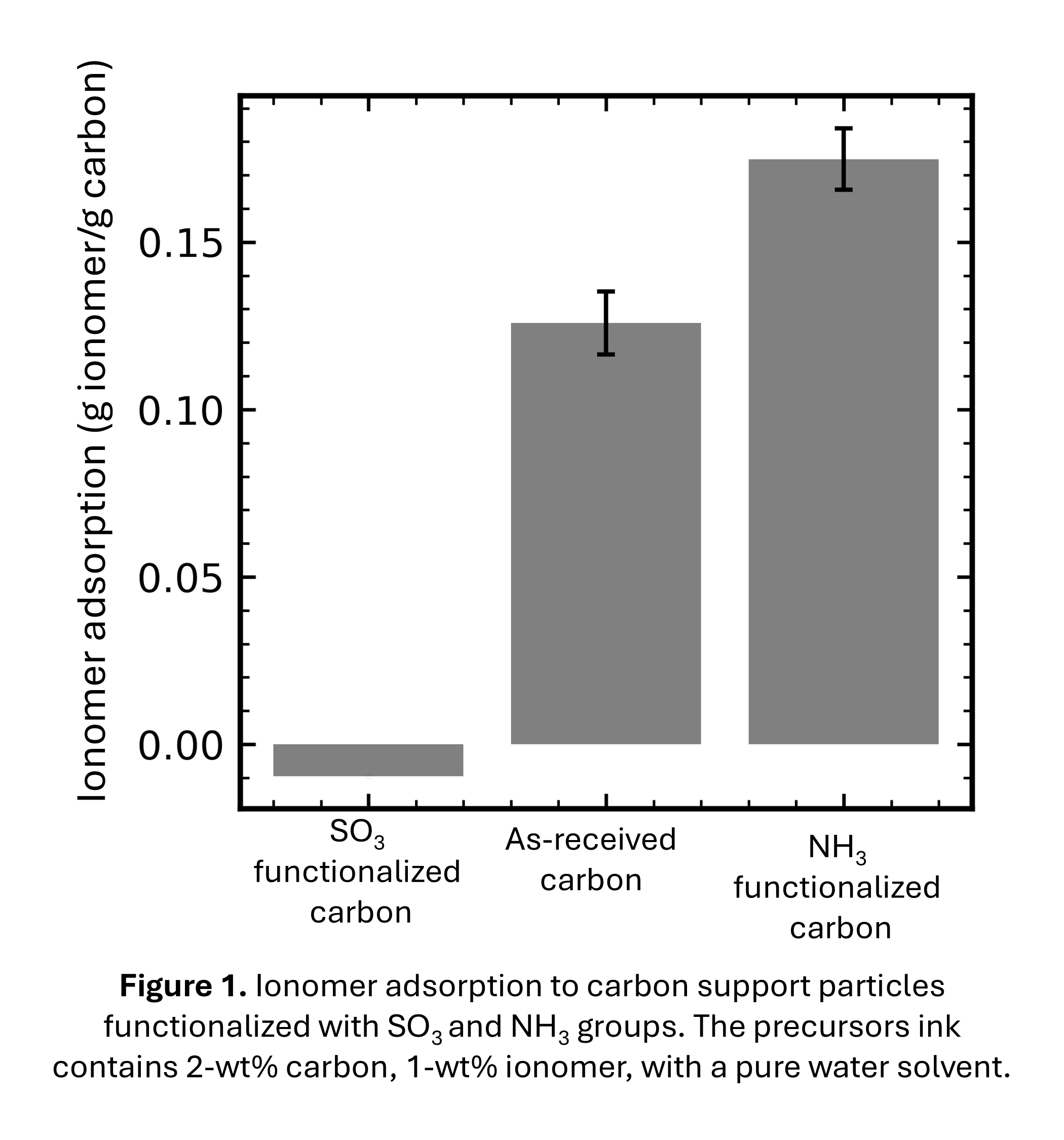
As shown in the figure below, functionalizing the carbon support significantly affects the ionomer adsorption. In particular, the extent of adsorption is governed by the carbon surface charge imparted by functional groups: SO3 and NH3 groups impart negative and positive charge respectively on the carbon surface. The anionic-sulfonic-acid moieties on the ionomer electrostatically repel the negatively charged carbon surface resulting in little adsorption and attract to the positively charged carbon surface. Comparing trends in ionomer adsorption and cell performance/diagnostics suggests that enhanced ionomer adsorption in the ink improves catalyst-layer ionomer distribution and performance.