2025 AIChE Annual Meeting
(333b) Ion Transport Limits Acid and Base Concentrations in Redox Electrodialysis for Environmental Separations
Authors
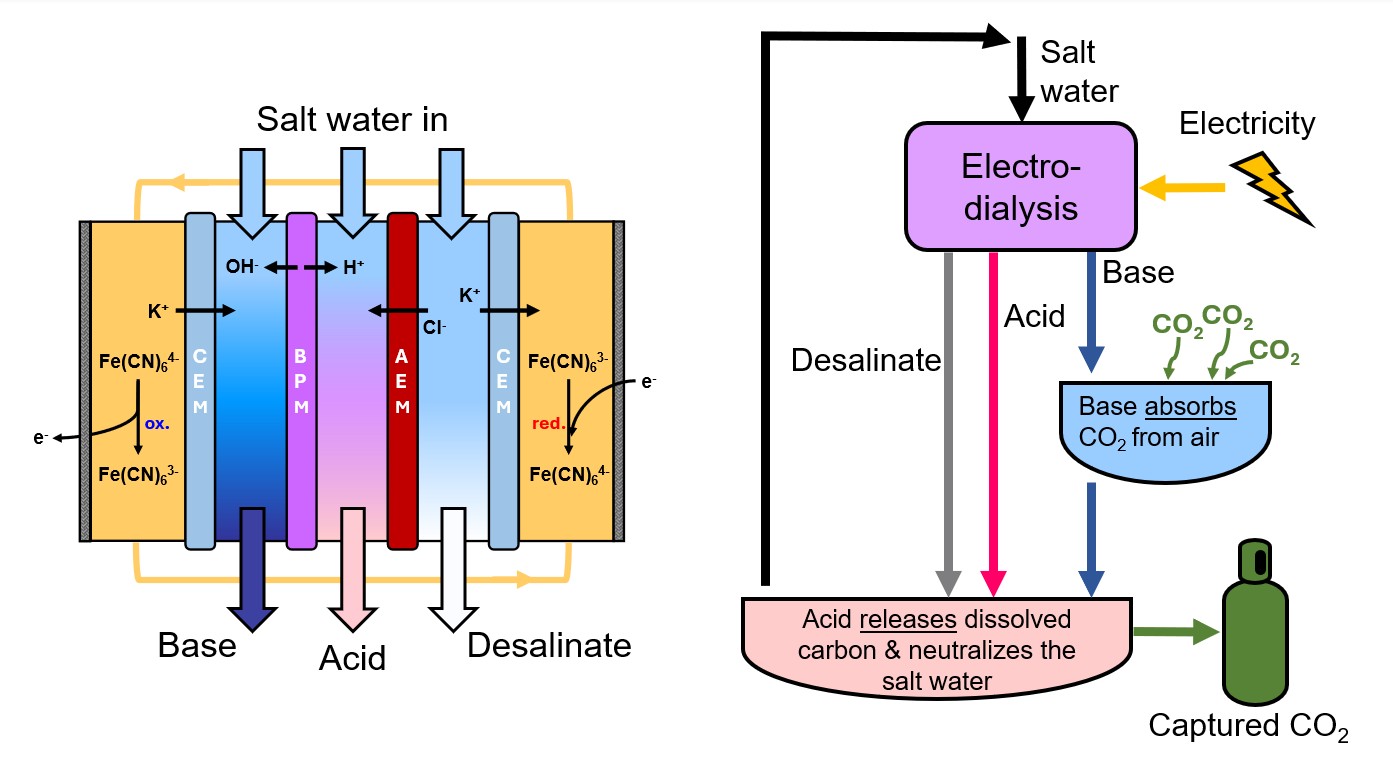
For direct air capture of carbon dioxide, BPMED may be used to generate an alkaline electrolyte that absorbs carbon dioxide from the air as dissolved inorganic carbon, and an acidic electrolyte to mix with the carbon-rich capture solution to release the captured carbon for permanent storage or use. The below figure illustrates a proposed alkalinity swing cycle based on these principles, as well as a schematic redox electrodialysis reactor.
The rate of carbon dioxide capture as well as the volumetric capacity for dissolved inorganic carbon depend on the concentrations of acid and base that can be generated in the redox electrodialysis reactor at acceptable energy cost. In this talk, we will discuss the voltage of our redox electrodialysis reactor and its contributing factors. Further, we will examine the effects of acid/base concentration and current density on the efficiency of acid/base generation. We will uncover the ion transport mechanisms responsible for current inefficiency, identifying that in our experiments, crossover of protons through the anion exchange membrane (AEM) limits the overall efficiency most of all. Finally, we will propose electrochemical engineering strategies toward high efficiency, low voltage generation of acid and base toward sustainable electrochemical separations.