2025 AIChE Annual Meeting
(382o) Ion Exchange of Zeolitic Brønsted Acid Sites with Metal Cations Influences the Hydrocarbon Pools (HCP) during Tandem CO2 hydrogenation
Authors
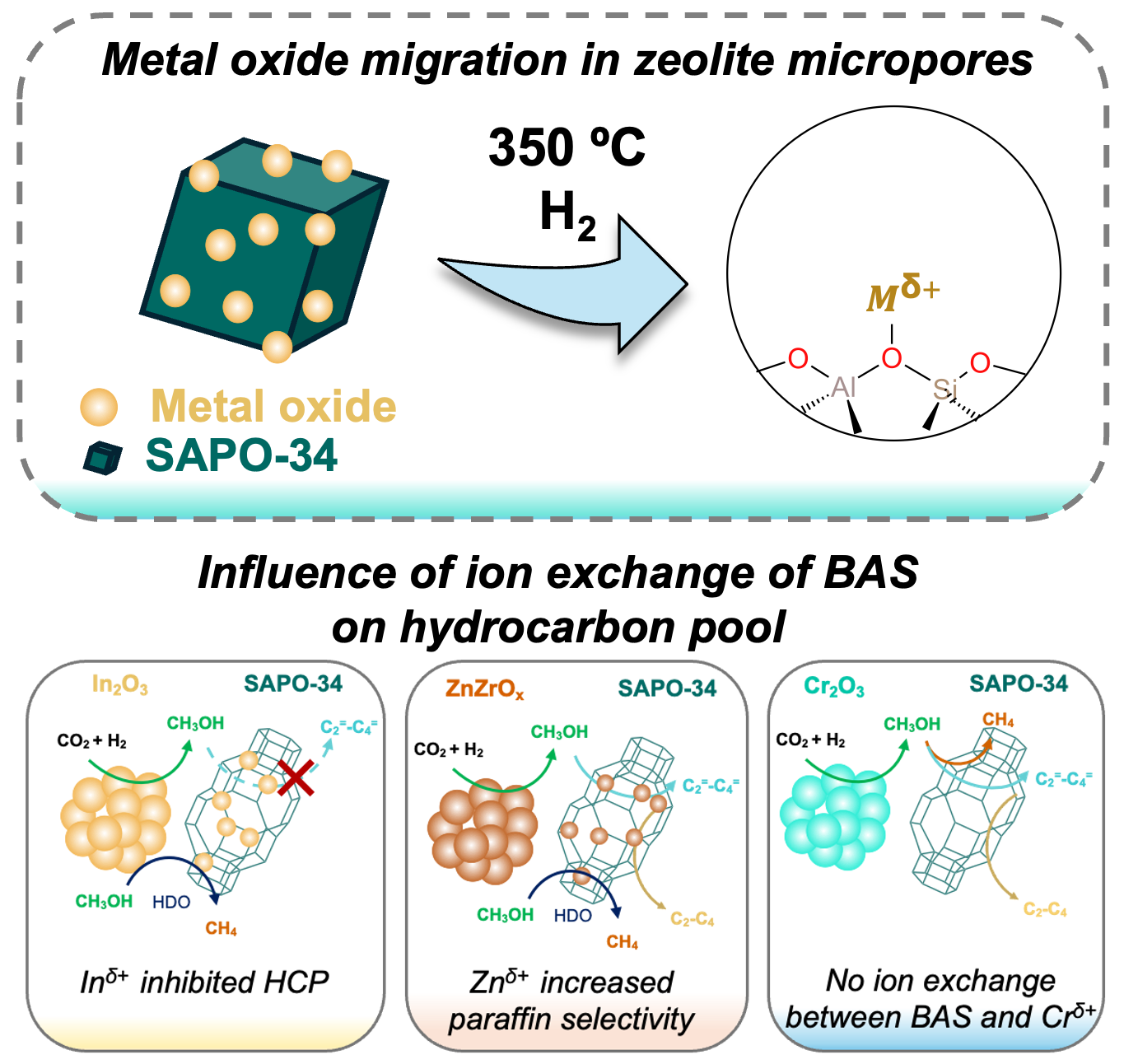
We demonstrate that the exchange of zeolitic Brønsted acid sites (BAS) with cations from metal-oxides plays a pivotal role in the propagation of hydrocarbon-pools (HCP) during tandem CO2 hydrogenation. We probed the likelihood of In2O3, ZnZrOx, and Cr2O3 migration, and their cation exchange with BAS of a silicoaluminophosphate, SAPO-34 by integrating them at nanoscale proximity. Analysis with NH3-temperature programmed desorption and transmission Fourier transform infrared spectroscopy showed ion-exchange of BAS with Inδ+ and Znδ+, but not for Crδ+. We measured C3/C2 hydrocarbon ratio (indicates relative propagation of olefin to aromatic cycles) and paraffin-to-olefins ratio (indicates degree of saturation of hydrocarbons), which revealed that Inδ+ species inhibited HCP inside the channels of SAPO-34 while Znδ+ species enhanced hydrogen transfer and secondary hydrogenation. Combining reactivity data with occluded hydrocarbon analysis and 13C solid-state nuclear magnetic resonance spectroscopy, we show that ion-exchanged species affect HCP propagation. Overall, this study offers valuable insights into the nuanced influence of different metal oxide migration and their cation exchange with BAS on the catalytic performance of bifunctional oxide/zeolite systems. Particularly, in the context of the HCP mechanism, these findings contribute to a deeper understanding of tandem CO2 hydrogenation over diverse oxide/zeolite systems.
Research Interests
I aim to develop sustainable processes to address climate challenges and produce fossil-fuel alternatives through innovative reactor and catalyst design strategies. The following sections summarize my previous, current and future research directions.
Previous Research
My PhD research focuses on the catalytic tandem conversion of carbon dioxide (CO2) into value-added fuels and chemicals utilizing bifunctional metal-oxide/zeolite catalysts. The tandem reaction integrates methanol synthesis on redox sites of metal oxides with methanol-to-hydrocarbon conversion on Brønsted acid sites (BAS) of zeolites. The reaction is attractive as it promotes process intensification, reducing capital and operational costs by consolidating multiple reaction steps in a single reactor, making it particularly attractive for hard-to-electrify sectors like sustainable aviation fuel (SAF) production.1
Although the conversion of CO2 to higher hydrocarbons seems appealing, the molecular underpinnings of the complex interactions of different active sites with the reactants and intermediates during the reaction is not well understood. While it is widely believed that bringing catalytic sites closer together (e.g., redox and acid sites), improve reaction rates and selectivity toward desired hydrocarbons (HC), our findings challenge this hypothesis by showing that there is a tradeoff: increasing active site proximity does not always lead to better outcomes.2 Specifically, we probed that increasing the proximity between In2O3 and HZSM-5 from the millimeter to the micrometer scale resulted in an eight-fold increase in C–C coupled products by facilitating intermediate methanol transport and conversion, whereas nanoscale proximity induced undesirable metal oxide migration into the zeolite framework and consequently exchanged acid sites, suppressing hydrocarbon formation.3, 4 We further unraveled how the migration of different metal oxides (e.g., In2O3, ZnZrOx, Cr2O3 etc.) inside zeolitic pore channels and their cation exchange with acid sites influence the HC formation mechanism.5 Specifically, we probed while ion-exchanged Inδ+ species inhibit HC formation and propagation, Znδ+ species enhance the selectivity and yield of paraffins by increasing hydrogen transfer mechanism over Znδ+ exchanged sites. Overall, my research established design principles for spatial integration of redox and acid sites in bifunctional systems and provided critical insights into the nuanced influence of diverse oxide migration inside zeolite micropores on hydrocarbon formation mechanism.
Current Research
Currently, I am investigating to unravel the coupled impact of reaction kinetics and mass‐transport phenomena on HC formation rates and selectivity during CO2 hydrogenation to define the poorly investigated mechanistic basis of the site-proximity effect. To elucidate the diffusion and transport artifacts in this chemistry, I aim to probe i) how diffusion constraints imposed by microporous zeolites influence reaction mechanism and HC formation rates, and ii) develop a proximity criterion for avoiding transport and diffusion limitations. By elucidating the mass transport effects in this chemistry, my research will unravel the molecular underpinnings of site-proximity effects during CO2 hydrogenation. I further aim to develop stable and selective catalysts for efficient CO2 conversion by designing core-shell catalysts to prevent the migration of metal oxides inside zeolite framework. We aim to synthesize inert and non-polar silicalite-1 shell over metal oxide catalyst (e.g., In2O3@S-1) to create a diffusion barrier for i) inhibiting the thermal migration of oxide particles, ii) reducing moisture-assisted ion exchange of BAS, and iii) preventing deactivation and over-reduction of metal oxides in the presence of H2. Our preliminary data revealed that In2O3@S-1 core–shell catalyst maximizes HC yields with minimal deactivation from ion exchange. Overall, my research will guide us towards the optimal strategy for thermo-catalytic CO2 conversion which will have substantial impact on the sustainable energy landscape.
Future Direction
Moving forward, I aim to: i) develop strategies for designing multifunctional single-atom catalysts for heterogenous tandem hydrogenation and carbonylation of CO2 to acetic acid, ii) design modular Joule-heated reactors for distributed propylene and syngas production directly at shale-gas sources, and iii) harness pH-dependent spontaneous polarization at metal-support interfaces to tune interfacial electric fields, overcoming activity–selectivity trade-offs in acetylene hydrogenation. Achieving these aims will position me to deliver transformational advances in heterogeneous catalysis and reaction engineering field—from pioneering carbon-negative routes for chemical synthesis to developing reactor technologies that are modular, electrified, and tailored for facilitating sustainable processes.
Teaching Interest
I am deeply passionate about creating an interactive and student-centered learning environment that promotes curiosity, critical thinking, and collaboration. My teaching interests lie primarily in core chemical engineering courses such as Kinetics and Reactor Design, Mass Transfer, and Transport Phenomena— where abstract concepts often challenge students and demand creative instructional approaches.
As a teaching assistant for Kinetics and Reactor Design course, I observed that students often struggled with conceptual problems. To address this, I integrated tools like Kahoot to transform multiple-choice questions into dynamic quizzes that sparked interest and participation. I also led weekly recitation sessions and encouraged small-group discussions to foster peer-to-peer learning.
In addition, my role as a Graduate Teaching Consultant (GTC) at Texas A&M’s Center for Teaching Excellence (CTE) has significantly shaped my approach. Through workshops and consultations with instructors across disciplines, I gained insight into evidence-based pedagogical strategies and developed a broader perspective on how to assess and support student learning. This experience has equipped me to reflect critically on my own teaching, adapt to diverse learning needs, and mentor others in improving their instructional practices. My aim is to cultivate an environment where learning is not only rigorous but also enjoyable and inclusive.
Selected Publications
- Mahnaz, F.; Dunlap, V.; Helmer, R.; Borkar, S. S.; Navar, R.; Yang, X.; Shetty, M. ChemCatChem 2023, 15 (17), e202300402.
- Mahnaz, F.; Iovine, A.; Shetty, M. Sci. 2025, 16, 10106–10118.
- Mahnaz, F.; Mangalindan, J. R.; Dharmalingam, B. C.; Vito, J.; Lin, Y.-T.; Akbulut, M.; Varghese, J. J.; Shetty, M. ACS Sustain. Chem. Eng. 2024. 12 (13), 5197-5210.
- Mahnaz, F.; Mangalindan, J.; Vito, J.; Helmer, R.; Shetty, M. Catal. 2024, 434, 115518.
- Mahnaz, F.; Dharmalingam, B. C.; Mangalindan, J. R.; Vito, J.; Varghese, J. J.; Shetty, M. Chem Catal. 2024. 5 (2), 101183.
Awards
- Texas A&M Chevron Energy Graduate Fellowship, 2025-2026: Awarded for excellence in energy-related research, sponsored by Chevron.
- 74th Lindau Nobel Laureate Meeting, Germany, 2025: Selected as a Lindau Scholar to participate in the premier meeting that brings together 35 Nobel Laureates and 600 Young Scientists from all over the world. Expenses covered by Lindau Foundation.
- Richard J. Kokes Award for North American Meeting (NAM 29), 2025
- Travel Award for North American Symposium for Chemical Reaction Engineering, 2025
- Global Young Scientists Summit, GYSS, Singapore, 2025: Selected to participate in the premier event that brings together Nobel Laureates, Field Medalists, Turing Fellows and ‘the brightest emerging minds in science from around the world’. All expenses covered by National Research Foundation (NRF).
- Dow Chemical Grad Fellowship by Department of Chemical Engineering, 2024: Awarded to a top student among ~135 graduate students in the Department of Chemical Engineering in recognition of excellence in research.
- Graduate Teaching Consultant (GTC) at the Center of Teaching Excellence (CTE), TAMU, 2024: Selected as a GTC for training and preparing teaching assistants for classroom teaching by professional development workshops and seminars.
- Best Poster Award at 2024 South West Catalysis Society Spring Symposium, 2024
- TAMU Graduate & Professional School Travel Award for 2023 AIChE Annual Meeting, 2023
- Catalysis and Reaction Engineering (CRE) Travel Award for 2023 AIChE Annual Meeting, 2023
- TAMU Chemical Engineering Ph.D. Qualifying Examination Excellence Award, 2022