2025 AIChE Annual Meeting
(118g) Investigating the Role of Brønsted Acid Sites and Co-Adsorbates on the Diffusion of C6–C12 Methylbenzenes in H-MFI Zeolite
Authors
Reilly Afflerback, University of Florida
Mykela DeLuca, University of Florida
David Hibbitts, University of Florida
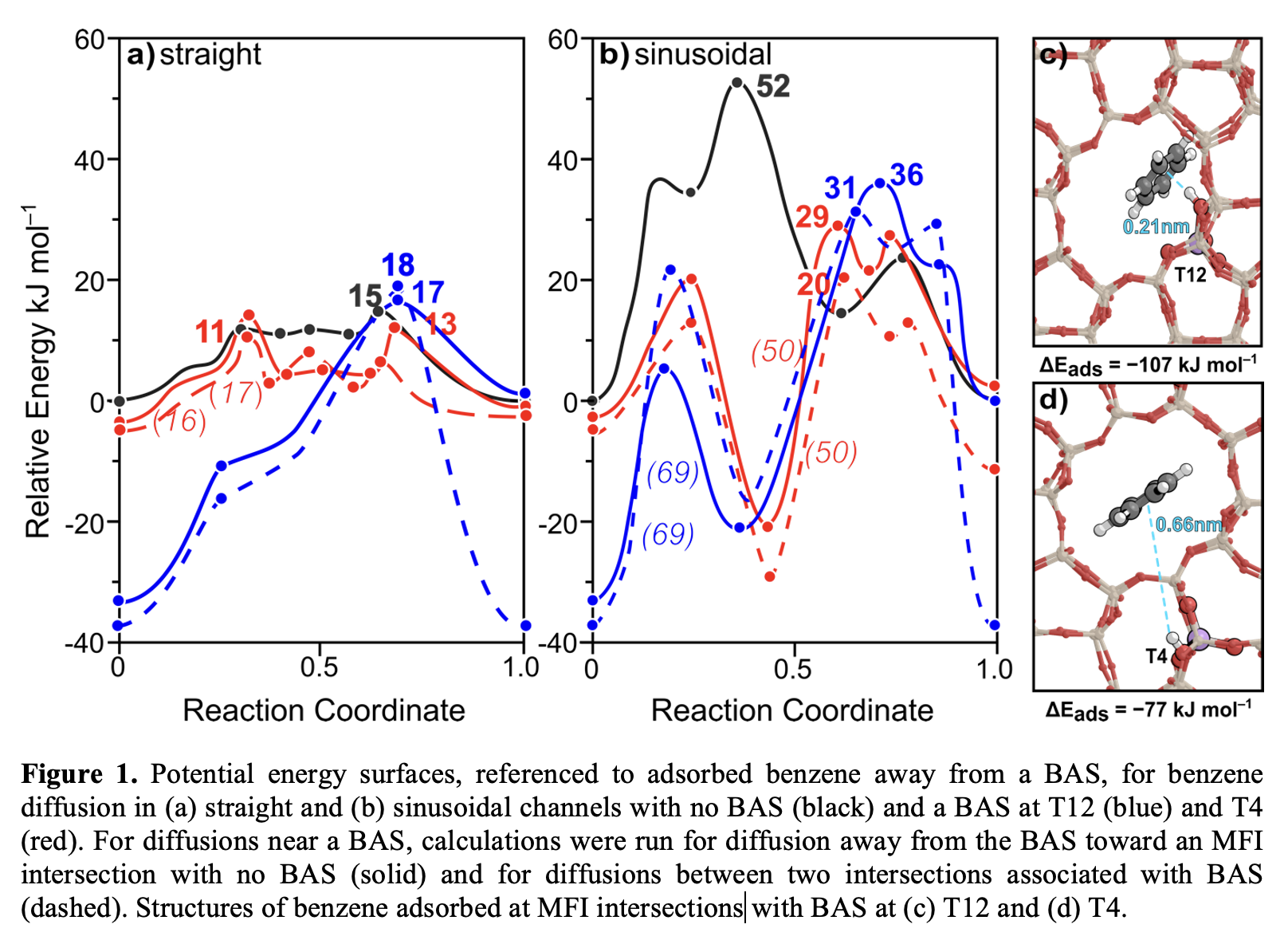
MFI-form zeolites enable shape-selective catalysis, where catalytic activity is influenced by both reaction kinetics and mass transport constraints imposed by the MFI framework. For example, in toluene methylation, MFI zeolites exhibit high para-xylene selectivity (30–99%) resulting from the favorable ease of diffusion of p-xylene compared to other isomers. Intracrystalline diffusion determines access to active sites in zeolite-catalyzed reactions involving aromatics, impacting overall reaction rates and selectivity. Thus, understanding the diffusion patterns of aromatic species within zeolite crystals is critical for elucidating the role of mass transport and catalyst deactivation. Previous work on the diffusion of C6–C12 methylbenzenes in Si-form MFI found that diffusion barriers for these species are consistently lower for straight channel diffusion than that of sinusoidal channel diffusion counterparts, and that these barriers correlate with molecule critical diameter. Here, we employ periodic density functional theory (DFT) to model how Brønsted acid-sites (BAS), co-adsorbed species, and intrazeolite concentrations influence diffusion barriers and diffusivities of mobile aromatics (e.g., toluene and pX). Results show that diffusing away from a BAS at T12, closest to the intersection (Fig.1c) through the straight channel increases the barrier from 15 to 50 kJ mol–1 because of the strong stabilization of the reactant via hydrogen bonding, (Fig.1) while the transition state remains unstabilized. The product is stabilized when diffusing between two BAS, but the barrier remains high (56 kJ mol–1) as the transition state is still distant from either BAS. A BAS at T4, farther from the intersection (Fig.1d) has a negligible impact on straight-channel diffusion. In the sinusoidal channel BAS at T12 raises the barrier from 52 to 69 kJ mol–1 by preferentially stabilizing the reactant. A BAS at T4 stabilizes both the bound and transition states by ~20 kJ mol–1, resulting in similar barriers to the Si-form.