2025 AIChE Annual Meeting
(318h) Investigating the Production, Characterization, and Ecological Risk Assessment of Valuable Products from Pyrolysis of Paper Waste
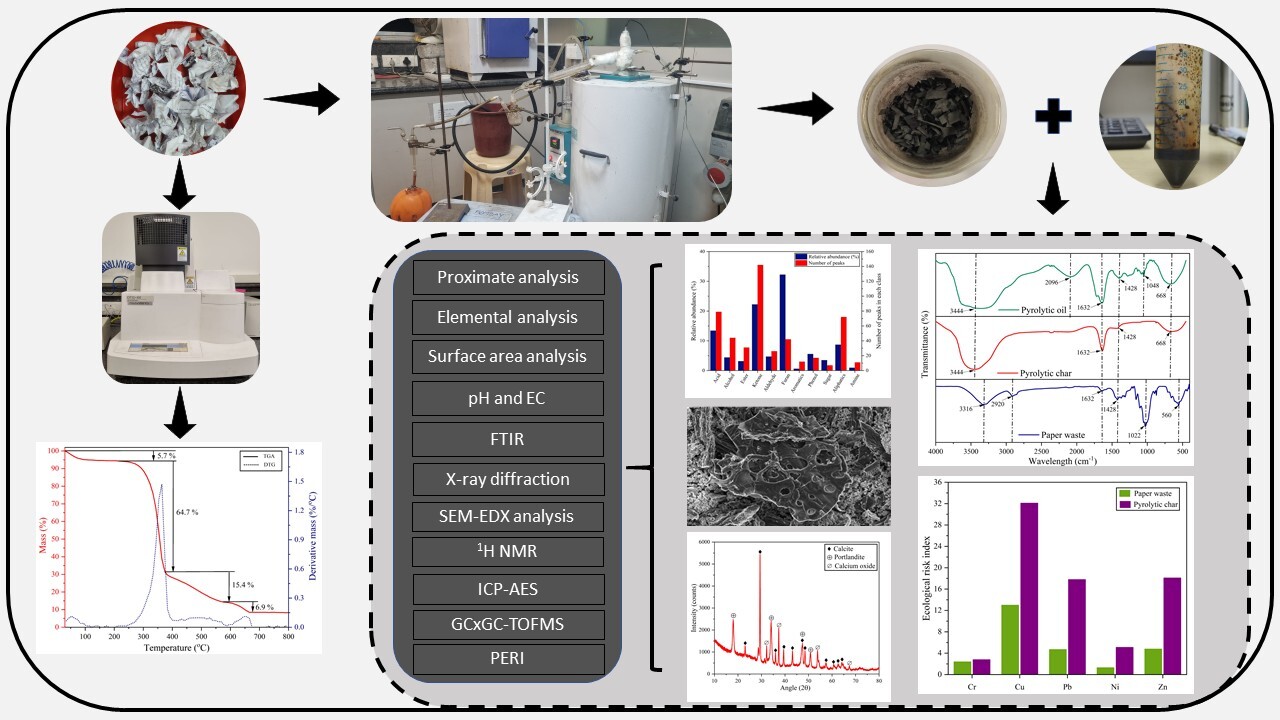
The pyrolysis of paper waste offers a promising approach to waste management and energy recovery. However, its valorization potential via pyrolysis remains underexplored in the Indian context. This study investigates the pyrolytic products derived from paper waste and assesses the environmental implications of heavy metals in the pyrolytic products. Thermogravimetric analysis was performed at a heating rate of 10 °C min-1 across a temperature range of 30–800 °C to determine the thermal degradation behavior. Based on these findings, batch pyrolysis experiments were conducted at 600 °C, yielding 25.8 wt.% char, 48.2 wt.% oil, and 26.0 wt.% gas. The products were characterized using proximate and elemental analysis, FTIR spectroscopy, SEM-EDS, BET surface area analysis, pH and EC measurements, XRD, GC×GC-TOFMS, 1H NMR, and ICP-AES. FTIR analysis indicates that the pyrolysis of paper waste results in significant chemical transformations. The pyrolytic oil was found to be rich in oxygenated species, including furans, phenols, ketones, and carboxylic acids, which affect its stability and necessitate upgrading for fuel applications. However, GC×GC-TOFMS and 1H NMR analyses suggest its potential for synthesizing high-value chemicals such as phenolic resins, ethanol, and lipids, along with selective production of olefins and aromatics. Heavy metal analysis via ICP-AES confirmed that pyrolysis effectively immobilizes metals in the char, with no detectable heavy metals in the oil. However, the char retained significant levels of copper (Cjf=6.4, 6<Cjf≤9, moderate contamination) and zinc (Cjf=18.1, Cjf>9, high contamination), limiting its direct application as a soil amendment. This study provides a comprehensive chemical composition analysis of pyrolytic products, essential for optimizing the pyrolysis process and assessing the potential for producing value-added chemicals and energy recovery from paper waste.