2025 AIChE Annual Meeting
(379g) Investigating PFOA Sorption on Silica with Time-Domain NMR
One mitigation strategy lies in the development of methods to remove PFAS from the environment, and particularly water. PFAS-contaminated water sources pose a direct risk to public health, as drinking water is a primary exposure route. Techniques for the removal of PFAS from water are well reviewed7 and include sorption,8,9 membrane separation technology,10,11 fractionation,12,13 and destruction.14 Sorption is a highly effective method for the removal of long chain PFAS where stronger Van Der Waals interactions enhance the binding strength between PFAS and the sorbent.
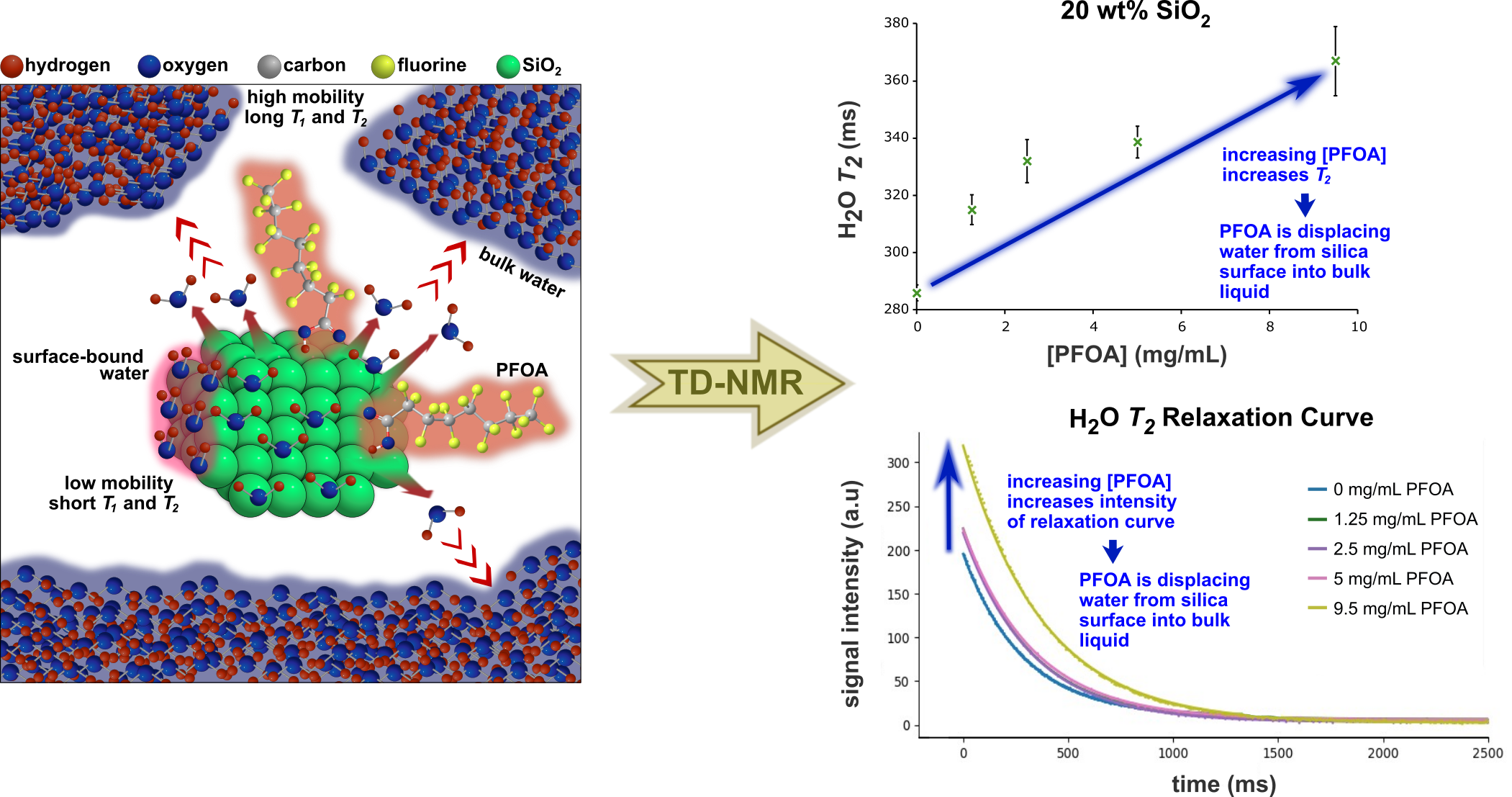
Presented here is an evaluation of liquid-phase proton NMR relaxometry as a technique for screening and monitoring porous and non-porous materials for PFAS sorption in water. Time-Domain (TD)-NMR offers two key advantages for this application: (1) TD-NMR systems are affordable and compact, and (2) samples can be analysed under wet conditions, enabling the evaluation of adsorbent performance under conditions that more closely resemble real-world scenarios. NMR relaxation is highly sensitive to the local chemical and magnetic environment of the nuclei under investigation. As a result, relaxation measurements of solvent nuclei in a sample containing a dispersion of the adsorbent candidate in the presence of PFAS, can indirectly probe PFAS-adsorbent interactions. This is because PFAS adsorption displaces solvent molecules from the adsorbent surface into the bulk liquid. Since NMR relaxation times are proportional to molecular mobility, this displacement increases the solvent population in the bulk liquid, where molecular mobility is significantly higher than that of surface-bound solvent molecules, leading to an increase in the measured relaxation time. Furthermore, the magnitude of this increase correlates with the extent of PFAS sorption. Thus, NMR relaxometry provides valuable insight into adsorption strength and dynamics and provides a cost-effective and time-efficient method for evaluating sorbent performance under application conditions.
This study interrogated the sorption of perfluorooctanoic acid (PFOA) onto a non-porous silica dispersed in water. Spin-lattice (T1) and spin-spin (T2) relaxation times of water were measured via Inversion Recovery (IR) and Carr-Purcell-Meiboom-Gill (CPMG) pulse sequences, respectively, across samples with varying PFOA (0, 1.25, 2.5, 5 and 9.5 mg/mL) and silica (5, 10, 20 and 40 wt%) concentrations. Following the addition of PFOA, an increase in the T1 and T2 relaxation curve intensities was observed at all silica concentrations, indicating a rise in bulk water content that confirms the displacement of silica-bound water molecules by adsorbed PFOA. Additionally, relaxation times increased with PFOA concentration at silica concentrations of 5, 20 and 40 wt% for T1, and at 20 and 40 wt% for T2. This confirms the increase in bulk liquid water within the sample because of water displacement by PFOA sorption. However, the absence of a consistent positive correlation between water relaxation times and PFOA concentration across all silica concentrations is unclear. We hypothesize that this may be due to the negative correlation observed for increasing PFOA concentration in water. This opposing effect is more pronounced at lower silica concentrations, where fewer water molecules are bound to the silica surface and subsequently displaced, resulting in a weaker positive contribution to T1 and T2 as a function of PFOA concentration.
Future work will focus on relaxation measurements of water in porous silica suspensions, where a significantly larger surface area is expected to increase PFOA sorption, leading to greater changes in relaxation times that may minimise opposing contributions.
While further research is needed to fully establish NMR relaxometry as a screening tool for PFAS sorbents in water, these preliminary findings highlight the capability of NMR relaxometry to monitor PFAS sorption and qualitatively assess sorbent performance by comparing relaxation time changes across different sorbate and sorbent concentrations.
References
1 J. C. Botelho, K. Kato, L.-Y. Wong and A. M. Calafat, Environ. Res., 2025, 270, 120916.
2 S. E. Fenton, A. Ducatman, A. Boobis, J. C. DeWitt, C. Lau, C. Ng, J. S. Smith and S. M. Roberts, Environ. Toxicol. Chem., 2021, 40, 606–630.
3 S. Li, P. Oliva, L. Zhang, J. A. Goodrich, R. McConnell, D. V. Conti, L. Chatzi and M. Aung, J. Expo. Sci. Environ. Epidemiol., 2025, 1–12.
4 K. E. Pelch, A. Reade, T. A. M. Wolffe and C. F. Kwiatkowski, Environ. Int., 2019, 130, 104851.
5 O. US EPA, Our Current Understanding of the Human Health and Environmental Risks of PFAS, https://www.epa.gov/pfas/our-current-understanding-human-health-and-env…, (accessed 20 March 2025).
6 Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS), https://www.niehs.nih.gov/health/topics/agents/pfc, (accessed 20 March 2025).
7 R. Amen, A. Ibrahim, W. Shafqat and E. B. Hassan, Sustainability, 2023, 15, 16173.
8 E. Gagliano, M. Sgroi, P. P. Falciglia, F. G. A. Vagliasindi and P. Roccaro, Water Res., 2020, 171, 115381.
9 C. T. Vu and T. Wu, Crit. Rev. Environ. Sci. Technol., 2022, 52, 90–129.
10 T. Lee, T. F. Speth and M. N. Nadagouda, Chem. Eng. J., 2022, 431, 134023.
11 S. Das and A. Ronen, Membranes, 2022, 12, 662.
12 H. Pang, B. Dorian, L. Gao, Z. Xie, M. Cran, S. Muthukumaran, F. Sidiroglou, S. Gray and J. Zhang, Sci. Total Environ., 2022, 827, 154310.
13 T. Buckley, K. Karanam, H. Han, H. N. P. Vo, P. Shukla, M. Firouzi and V. Rudolph, Water Res., 2023, 230, 119532.
14 J. N. Meegoda, B. Bezerra de Souza, M. M. Casarini and J. A. Kewalramani, Int. J. Environ. Res. Public. Health, 2022, 19, 16397.