2025 AIChE Annual Meeting
(441g) Investigating Hydrogenative Debenzylation: Mechanistic Modeling to Enhance Understanding of Scale-Dependent Catalyst Deactivation
Authors
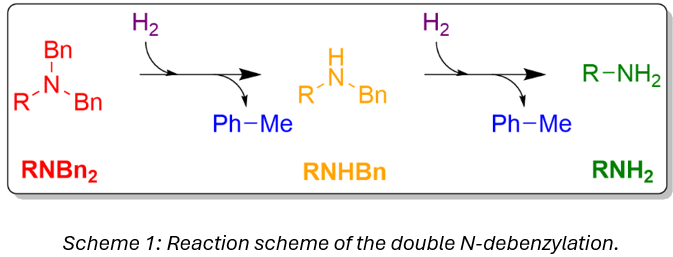
Deprotection of N-benzyl protecting groups is commonly performed by catalytic hydrogenolysis.1,2 The system under investigation involves a two-step debenzylation, going through a monobenzyl intermediate (Scheme 1). This is a key step in the synthesis of an active pharmaceutical ingredient The manufacturing process of this reaction has experienced significant variability in the reaction time required to pass the in-process control (IPC). While normally held for 24 hours, the reaction has been observed to take up to 175 hours to complete. The long and uncertain reaction time makes reliable resource and timeline planning intractable in manufacturing. This work aimed to identify and characterize the source of the process variability through in silico hypothesis testing and experimental validation.3
Methods
A thorough mapping of the gas-liquid mass transfer coefficient (kLa) was acquired in an EasyMax reactor. Early experiments demonstrated strong sensitivity to the stir speed. While the rate of the first debenzylation aligns with the kLa mapping, indicating gas-liquid mass transfer limitations, the rate of the second debenzylation was suppressed by orders of magnitude with decreasing stir speed. To further explore this observation, a design of experiment (DoE) was planned and executed to target three kLa values at three different volumes by modulating stir speed. In this way, the effects of kLa were decoupled from solid-liquid limitations.
Results
The results of the DoE demonstrate that the reaction rate at constant kLa but different stir speed is significantly different. The cases at the lowest stir speed showed substantial kinetic suppression, indicating that, despite being well dispersed, solid-liquid mass transfer limitations appear to contribute significantly to the long reaction times. Further experiments revealed that the observed effect is not reversible. After operating for some time under mass-transfer limited conditions, increasing the stir speed does not restore the usual reactivity. Model prediction of the catalyst surface composition indicates a peak in the product concentration early into the reaction, where the rate of production is significantly faster than the rate of transport away from the surface. The long residence time and high surface concentration appears to lead to catalyst poisoning.4 This is possibly through the migration of the product from a top site to a bridge site, where the adsorption energy is substantially increased.5
Implications
These findings provide a mechanistic understanding of how and why the large-scale process suffers from long and variable batch times. This insight can be leveraged to appropriately plan manufacturing operations. Particularly, mixing calculations can be employed to assess the suitability of reactors for this process. Additionally, sampling times can be appropriately established to identify issues and enable early supplementary catalyst charges. The identification of the solid-liquid mass transfer promoted catalyst poisoning also highlights significant opportunity for implementation of a continuous operation, where the improved mass transfer rate would protect against this phenomenon.
References
[1] David, A., Vannice, M. (2006). Control of catalytic debenzylation and dehalogenation reactions during liquid-phase reduction by H2. Catal., 237(2), 349–358.
[2] Smith, G. V., Notheisz, F. (1999). Chapter 4 – Hydrogenolysis. In Heterogeneous Catalysis in Organic Chemistry (pp. 119–218). Academic Press.
[3] Raymond, T., et al. (2024, October 27 – 31). Transport-kinetic modeling of a double N-debenzylation in the production of an active pharmaceutical ingredient [Conference presentation abstract]. 2024 AIChE Annual Meeting, San Diego, CA, United States. https://proceedings.aiche.org/conferences/aiche-annual-meeting/2024/pro…
[4] Hegedűs, L., & Máthé, T. (2002). Hydrogenation of pyrrole derivatives: Part V. Poisoning effect of nitrogen on precious metal on carbon catalysts. Applied Catalysis A: General, 226(1), 319–322. doi:10.1016/S0926-860X(01)00898-5
[5] Herron, J. A., Tonelli, S., & Mavrikakis, M. (2012). Atomic and molecular adsorption on Pd(111). Surface Science, 606(21), 1670–1679. doi:10.1016/j.susc.2012.07.003