2025 AIChE Annual Meeting
(309d) Investigating CO2 Fixation Via Electrochemical Carboxylation of Organic Halide Substrates
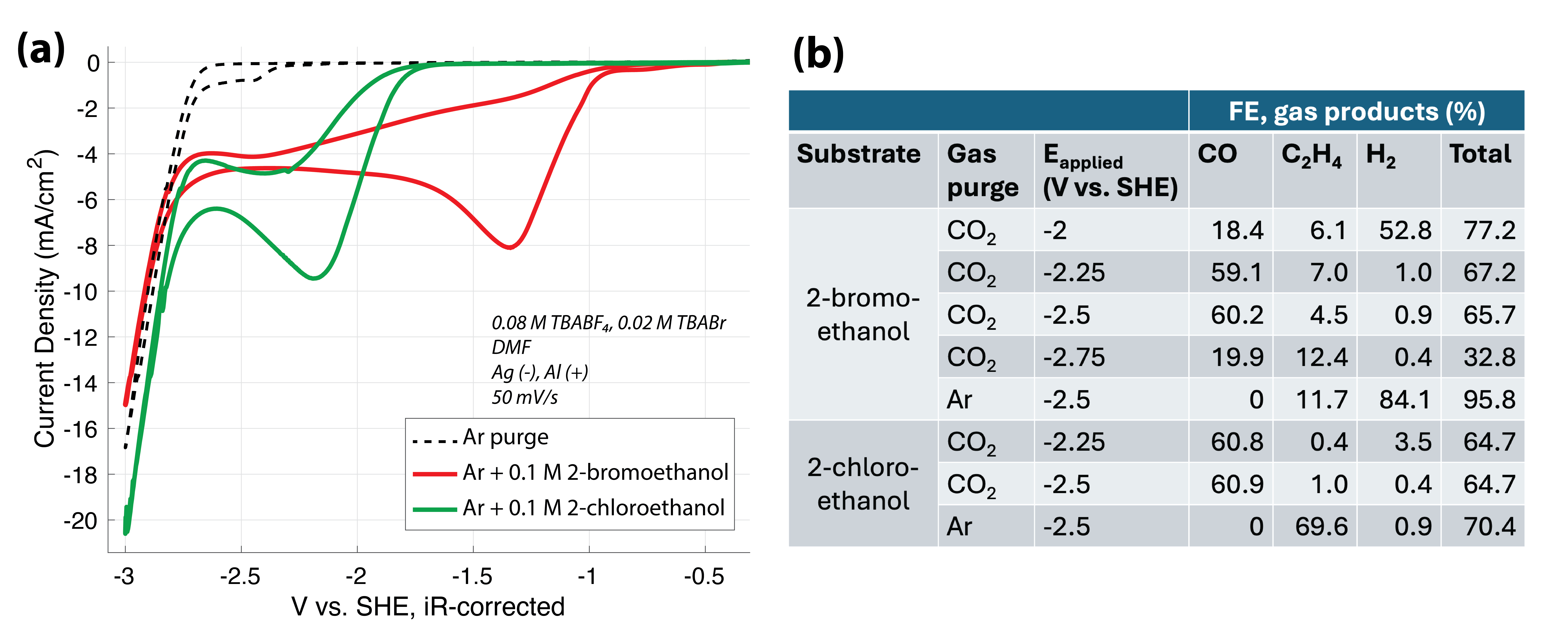
In this work, we present our investigation into CO2 coupling with ethylene halohydrin substrates over a heterogeneous electrocatalyst in polar aprotic electrolytes. We use transition and post-transition metal electrocatalysts as pure foils to elucidate reactivity trends. We select metals based on (1) their activity for direct substrate reduction and (2) whether they favor CO2 reduction (CO2R) towards CO/CO32- (e.g., Ag, Cu) or oxalate (e.g., Pb, Pt) in aprotic media. In this way, we elucidate how the metal identity tunes the selectivity of EC vs. CO2R and substrate reduction without CO2 coupling. We also confirm that the reduction potential of the halohydrin species is significantly modulated by halogen identity (i.e., Br, Cl) in aprotic media and that ethylene and CO are major electrolysis products over a Ag electrocatalyst, as shown in Figure 1.
Figure 1: (a) Representative cyclic voltammograms (CVs) for Ar-purged electrolyte with and without 0.1 M halohydrin substrates (2-bromoethanol or 2-chloroethanol) added. (b) Gas product quantification in terms of Faradaic efficiency (FE) using gas chromatography (GC). Gas purge flow rate = 20 sccm.