2025 AIChE Annual Meeting
(298d) Investigating the Behaviour of Promising Mixed Oxides for Key CO2 Adsorption Technologies
Gas-solid adsorption is a leading strategy to aid the production of clean fuels coupled with CCS. In this contribution, we investigate the suitability of promising mixed oxides to be used as CO2 sorbents in key sustainable technologies such as sorption-enhanced H2 production.[2,3] The materials explored include NaX zeolites, magnesium oxides (MgOs) and hydrotalcites (HTs), which exhibit attractive characteristics to adsorb CO2 in diverse operating windows and reaction environments required for such processes.[4]
The mixed oxides comprise commercial and as-synthesised adsorbents. The materials are characterised systematically using several physicochemical techniques. The CO2 adsorption-desorption isotherms of the solids at relevant temperature and pressure ranges (473-773 K, 1-10 bar) are carried out using primarily Thermal Gravimetric Analysis. The stability of the adsorbents is assessed by conducting pressure and temperature-swing cycles (PSA and TSA).
The results of this work show that, under typical conditions for sorption-enhanced H2 production, the HTs exhibit higher adsorption capacities per unit mass of sorbent at relatively low pressures and per unit surface area over the whole range of pressures analysed, compared to NaX zeolites. It is found that the MgOs assessed outperform these materials in terms of both types of capacity.
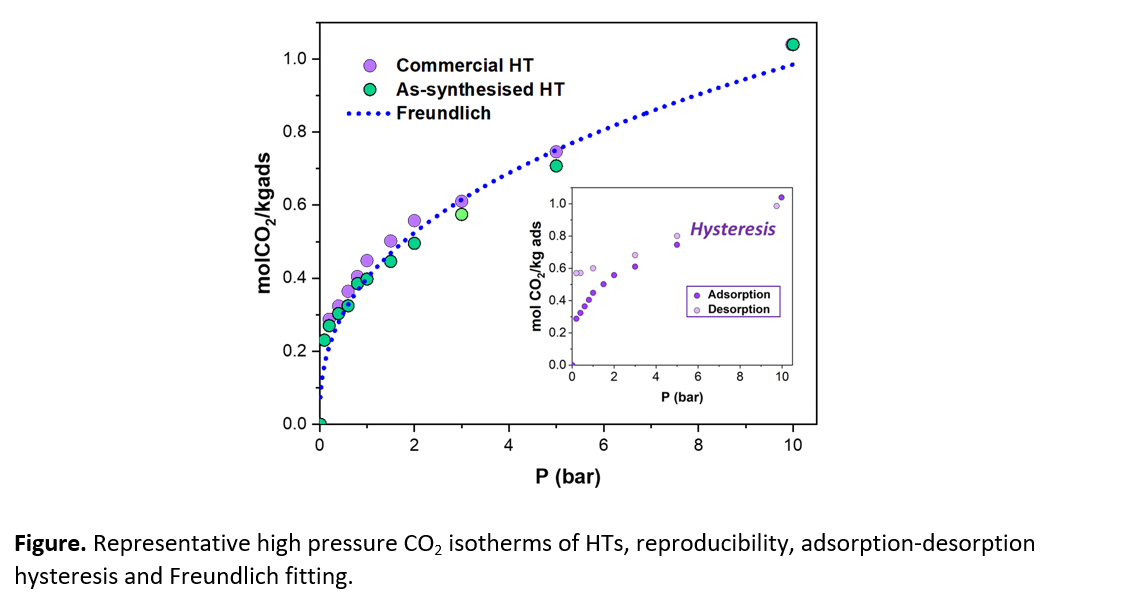
Hydrotalcites and also MgOs show adsorption-desorption isotherms with a hysteresis loop indicating a stronger binding energy with CO2, while the zeolites present a nearly reversible performance. In agreement with this, the NaX zeolites have a very stable performance upon cycling. On the other hand, HTs and MgOs exhibit a marked deactivation, which can be reduced by the use of carbon supports and alkali promoters.[5] In addition, PTSA regeneration appears to be more effective than PSA and TSA alone to mitigate the deactivation observed under the conditions assessed.
The equilibria data of the mixed oxides show good reproducibility and can be successfully fitted to Freundlich and Langmuir models. The values of heat of adsorption calculated for the fresh and cycled samples are useful input data for mathematical models of the sorption units.
References
[1] CO2 emissions in 2023, International Energy Agency report, 2024.
[2] D. Iruretagoyena, K. Hellgardt, D. Chadwick, Int. J. Hydrog.Energy, 43 (2018), p. 4211.
[3] J. Wong, D. Iruretagoyena, N. Shah, P. Fennell, Proc. R. Soc. A, 479, (2023) p. 20230173.
[4] D. Iruretagoyena, P. Fennell, R. Pini, Chem. Eng. J. Adv., 13, (2023) p. 100437.
[5] R. Menzel, D. Iruretagoyena, et al., Adv. Funct. Mater., 30, (2020) p. 2002788.