2025 AIChE Annual Meeting
(144j) Interplay of Ion-Ligand Affinity, Membrane Ligand Content, and Water Content on Ion Permeability
Authors
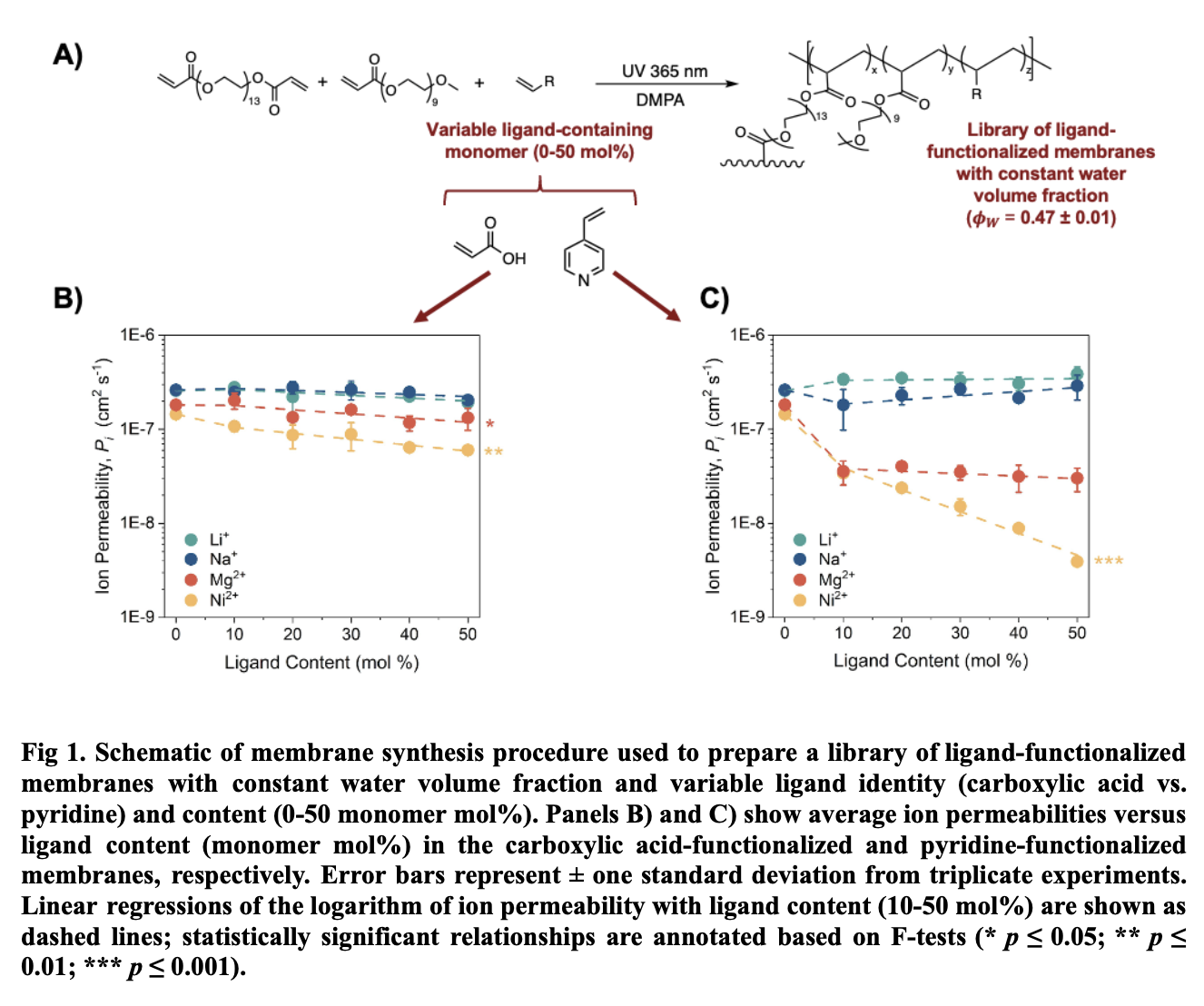
In this work, our fundamental studies of ion-transport across membranes with variable ligand species, ligand content, and water content serve to provide a clearer picture of membrane structure-function relationships pertinent to ion-selective separation applications. A systematic and comprehensive membrane synthesis and characterization workflow for the fabrication of polymeric membranes with controlled properties (e.g., chemistry, thickness, water content) was established for fundamental ion transport studies. The workflow comprises free-radical polymerization for membrane synthesis (Fig. 1A), along with gel fraction measurements, ATR-FTIR spectroscopy, and water uptake measurements for composition analysis. Membrane performance testing includes sorption measurements (to quantify ion partitioning), ion mobility measurements via electrochemical impedance spectroscopy, and ion permselectivity via diffusion and electrodialysis experiments in custom-made H-cells. These experiments were performed with electrolytes spanning a range of ligand affinities that are relevant to lithium-rich brine and battery leachate compositions (e.g., LiCl, NaCl, MgCl2, NiCl2, etc.).
Our work has demonstrated that not only ligand identity but also ligand content - a previously understudied parameter - are critical factors for ion permeability due to their combined effect on the overall ion-membrane affinity, specifically for ions capable of multidentate ligand complexation.5 For example, we observed an order-of-magnitude reduction in nickel permeability across membranes with increasing pyridine content (Fig 1C). As a result, we also observed increasing diffusive lithium/nickel permselectivity with increasing pyridine content in binary salt diffusion experiments. Coupled with ion partitioning experiments, we found that ion mobility effects dominate over partitioning effects in overall selectivity trends, aligning with prior theory suggesting that high-affinity, ligand-bound ions are relatively immobile within the membrane. As we gradually lower the membrane water content in these pyridine-functionalized membranes, preliminary results show further enhancement of lithium/nickel permselectivity. These results represent an order of magnitude improvement in diffusive lithium/nickel selectivity compared to commercially available cation exchange membranes - a meaningful enhancement for lithium battery recycling applications. Beyond the permselectivity improvement achieved in this work, the corresponding systematic analysis of membrane structure-performance relationships will contribute to our growing understanding of how membrane properties (ligand identity/content, water uptake, etc.) can be tuned to achieve desired ion transport properties for various ion-ion separations applications in the lithium supply chain and beyond.
References
(1) The National Academies of Sciences, Engineering, and Medicine. A Research Agenda for Transforming Separation Science; 2019. https://web-s-ebscohost-com.stanford.idm.oclc.org/ehost/ebookviewer/ebo… (accessed 2023-11-09).
(2) Cath, T. Y.; Chellam, S.; Katz, L. E.; Breckenridge, R.; Cooper, C.; Ellison, K.; Macknick, J.; McKay, C.; Miller, K.; Monnell, J.; Rao, N.; Rosenblum, J.; Sedlak, D.; Stokes-Draut, J. National Alliance for Water Innovation (NAWI) Resource Extraction Sector Technology Roadmap 2021; DOE/ GO-102021-5567; National Renewable Energy Lab. (NREL), Golden, CO (United States), 2021; pp 87–93. https://doi.org/10.2172/1782446.
(3) Zofchak, E. S.; Zhang, Z.; Marioni, N.; Duncan, T. J.; Sachar, H. S.; Chamseddine, A.; Freeman, B. D.; Ganesan, V. Cation–Ligand Interactions Dictate Salt Partitioning and Diffusivity in Ligand-Functionalized Polymer Membranes. Macromolecules 2022, 55 (6), 2260–2270. https://doi.org/10.1021/acs.macromol.2c00035.
(4) Marioni, N.; Nordness, O.; Zhang, Z.; Sujanani, R.; Freeman, B.D.; Segalman, R.A.; Clément, R.J.; Ganesan, V. Ion and Water Dynamics in the Transition from Dry to Wet Conditions in Salt-Doped PEG. ACS Macro Lett. 2024 13 (3), 341-347. https://doi.org/10.1021/acsmacrolett.4c00046.
(5) Abels, K.; Botelho Junior, A.B.; Chen, X.; Tarpeh, W.A. Ligand content and driving force effects on ion-ion permselectivity in ligand-functionalized membranes. J. Membr. Sci. 2025, 714, 123418. https://doi.org/10.1016/j.memsci.2024.123418.