2025 AIChE Annual Meeting
(111e) Interfacial Instability Due to Electrodeposition: Quasi 1-D Pattern Formation Validating a Theoretical Model
Authors
Alex M. Saperstein, University of Florida
Kirk Ziegler, University of Florida
Farzam Zoueshtiagh, Universite de Lille
Ranga Narayanan, University of Florida
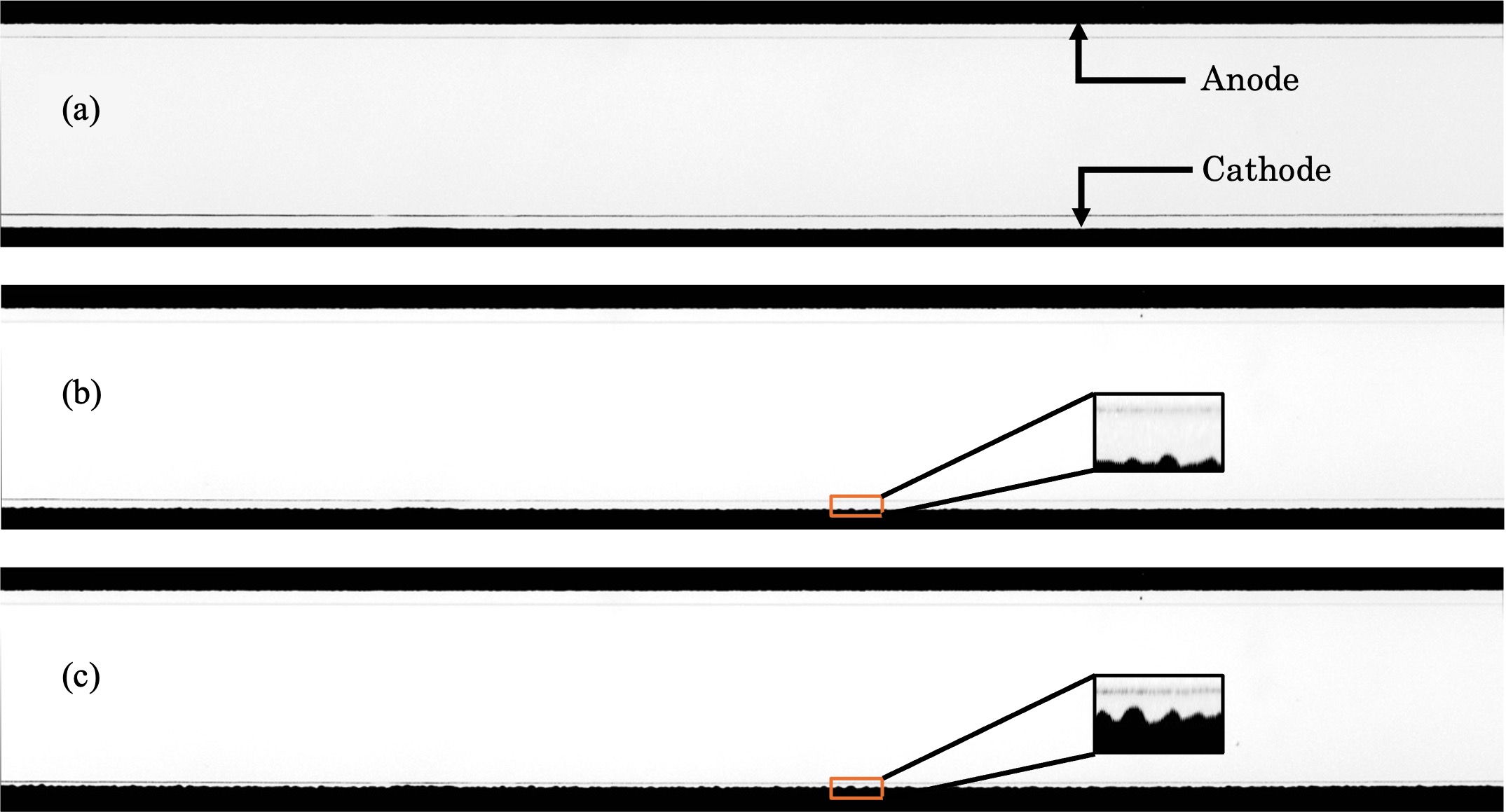
In this talk, results are reported from experiments that were conducted to investigate the structures formed at the cathodic surface when a small voltage was applied across an electrochemical cell composed of copper electrodes separated by a microfluidic channel filled with a dilute copper sulfate solution. The observed patterns were found to arise from an interfacial instability during electrodeposition, which is governed by a competition between the destabilizing effects of copper ion diffusion—driven by concentration gradients and the applied electric field—and the stabilizing effect of interfacial energy between the deposited copper and the surrounding electrolyte. Initially, cellular, wave-like structures were observed, which over time evolved into finger-like, mushroom-like, or branched dendritic forms. It was also observed that the characteristic wavelength of these structures was independent of the initial roughness of the electrodes. The electrochemical cells were fabricated using sputter deposition and photolithography in a manner that ensured growth occurred only on the cathode surface facing the anode. as shown in the accompanying diagrams. The measured wavelengths of the patterns were compared with predictions from an analytical result arising from an existing model (BuAli et al., 2006), which was developed under assumptions of local electroneutrality and negligible convection.
Figure: A typical electrodeposition experiment done at a constant input voltage. The output potential is higher at top electrode (anode) while it is lower at the bottom electrode (cathode). Images were taken at different times. (a) Initial flat surface. (b) Cellular formation starts which can be seen as roughening of cathode. (c) Cells continue to grow
Q.Buali, et. al. Electrochimica Acta, vol. 51, pp. 2881–2889, 3 2006.