2025 AIChE Annual Meeting
(479d) Integrated Solvent and Process Design of Crystallisation Processes with Solvent Recycling
The selection of an optimal solvent, solvent blend or antisolvent for a crystallisation process is challenging due to a vast number of possible solvent mixtures and the influence of solvent composition and the process conditions (e.g., crystallisation temperature) on the performance of the process. Furthermore, the optimal solvent / antisolvent pair and the optimal process conditions are interconnected and cannot be effectively determined separately2. To explore such an interconnected design space, solvent-based design methodologies have been employed to determine the optimal molecule (solvent), blend, process conditions or even the complete process configuration, satisfying the set of design constraints given one or more objective function and a mathematical model10. Typically, a CAMPD problem is framed as a mixed integer non-linear programming (MINLP) problem where discrete decision variables represent the choice of one or more solvents from a list of candidates or the selection of atom groups that make up the solvent(s)1. These problems are developed with aim of optimising specific process-based objectives such as maximizing the crystal yield3 or minimizing the solvent consumption4 and are notoriously difficult to solve due to the presence of these discrete decision variables and the highly nonlinear nature of the mathematical models.
However, the solution of the optimal design problem becomes even more complex when downstream solvent separation and recycling are considered. This complexity stems from the trade-off between the solvent properties that facilitate crystallisation and those required for efficient solvent separation and recycling1.
Various strategies have been proposed for optimal solvent selection and design of an isolated crystallisation process5-8. However, the solvent and process design problem for integrated crystallisation process with solvent recycling has received limited attention. Wang and Lakerveld1 focused on the solvent and process design for continuous antisolvent crystallisation integrated with a flash drum for solvent separation and recycling, where the temperature of crystalliser was set to a fixed value. A continuous mapping method was employed based on the perturbed-chain statistical associating fluid theory (PC-SAFT), thermodynamic model. Continuous model parameters were first optimised and the best match molecule for these parameters was then used to obtain the process conditions. This decomposition approach has the advantage of making the problem tractable but may lead to suboptimal results, since optimising molecular properties first may lead to poor compatibility with available solvents and process conditions, requiring additional adjustments. Moreover, the one-to-one mapping approach only associates solubility with a single solvent at a time and cannot incorporate solvent pair-dependent correction parameters, leading to less accurate solubility predictions for mixed solvent systems. Their results concluded that the proposed approach enables the systematic selection of solvent / antisolvent pairs that facilitate a sharp separation in the flash drum, making it easier to recycle the solvent and thereby reducing overall solvent consumption.
Recently, Watson et al.8 employed generalized disjunctive programming (GDP) within the CAMPD framework to develop a design formulation for hybrid cooling and antisolvent crystallisation process for ibuprofen and lovastatin. Further developing on their work, in this study, we present a comprehensive CAMPD formulation of a continuous crystallisation process for pharmaceutical products, with solvent / antisolvent separation and recycle.
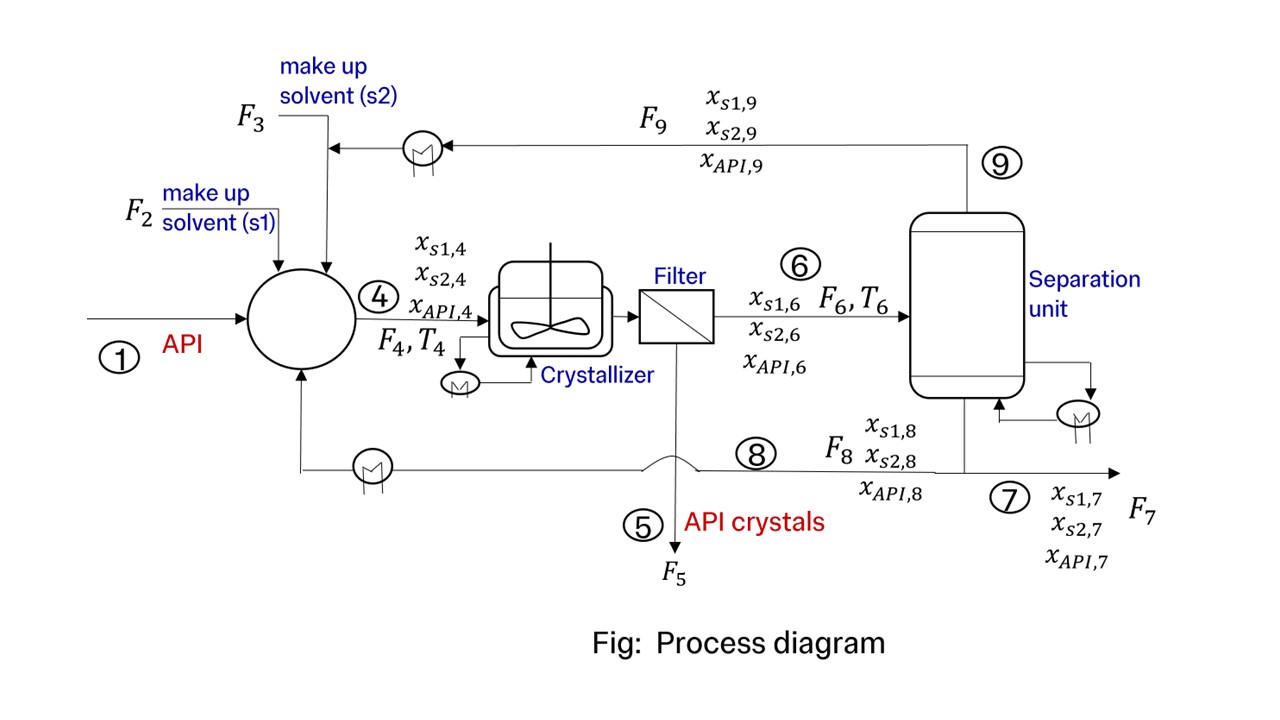
We specifically investigate the impact of recycling solvent and/or antisolvent on the process performance focusing on crystal yield, and process mass intensity. The proposed design formulation is flexible to allow for cooling, antisolvent, or hybrid crystallisation methods. Further, our methodology makes it possible to simultaneously identify the optimal solvent / antisolvent pair or the solvent mixture, the composition of the solvent mixture and process temperatures. To predict API solubility and other relevant thermodynamic properties, we employ the SAFT-γ Mie group-contribution-based thermodynamic model, which provides high-quality predictions of solubility in various solvents, as well as liquid-liquid and vapor-liquid equilibria--key properties for industrial applications8. The design formulation is implemented in gPROMS and is applied to crystallisation of mefenamic acid (MA), where the effect of crystallisation technique, process temperature and solvent specification are explored in the integrated crystallisation and solvent recycle framework.
References
- Wang, J. and Lakerveld, R., 2018. Integrated solvent and process design for continuous crystallization and solvent recycling using PC‐SAFT. AIChE Journal, 64(4), pp.1205-1216.
- Burger, J., Papaioannou, V., Gopinath, S., Jackson, G., Galindo, A. and Adjiman, C.S., 2015. A hierarchical method to integrated solvent and process design of physical CO 2 absorption using the SAFT‐γ M ie approach. AIChE Journal, 61(10), pp.3249-3269.
- Karunanithi, A.T., Achenie, L.E. and Gani, R., 2006. A computer-aided molecular design framework for crystallization solvent design. Chemical Engineering Science, 61(4), pp.1247-1260.
- Muhieddine, M.H., Viswanath, S.K., Armstrong, A., Galindo, A. and Adjiman, C.S., 2022. Model-based solvent selection for the synthesis and crystallisation of pharmaceutical compounds. Chemical Engineering Science, 264, p.118125.
- Karunanithi, A.T., Acquah, C., Achenie, L.E., Sithambaram, S. and Suib, S.L., 2009. Solvent design for crystallization of carboxylic acids. Computers & chemical engineering, 33(5), pp.1014-1021.
- Modarresi, H., Conte, E., Abildskov, J., Gani, R. and Crafts, P., 2008. Model-based calculation of solid solubility for solvent selection - A review. Industrial & engineering chemistry research, 47(15), pp.5234-5242.
- Sheikholeslamzadeh, E., Chen, C.C. and Rohani, S., 2012. Optimal solvent screening for the crystallization of pharmaceutical compounds from multisolvent systems. Industrial & engineering chemistry research, 51(42), pp.13792-13802.
- Watson, O.L., Jonuzaj, S., McGinty, J., Sefcik, J., Galindo, A., Jackson, G. and Adjiman, C.S., 2021. Computer aided design of solvent blends for hybrid cooling and antisolvent crystallization of active pharmaceutical ingredients. Organic process research & development, 25(5), pp.1123-1142.
- Wang, J. and Lakerveld, R., 2017. Continuous membrane-assisted crystallization to increase the attainable product quality of pharmaceuticals and design space for operation. Industrial & engineering chemistry research, 56(19), pp.5705-5714.
- Adjiman, C.S. and Galindo, A., 2025. Challenges and opportunities for computer-aided molecular and process design approaches in advancing sustainable pharmaceutical manufacturing. Current opinion in chemical engineering, 47, p.101073.