2025 AIChE Annual Meeting
(158e) Integrated Continuous USP Platform for Maximum Productivity and Closed-Loop Controlled CQA
Authors
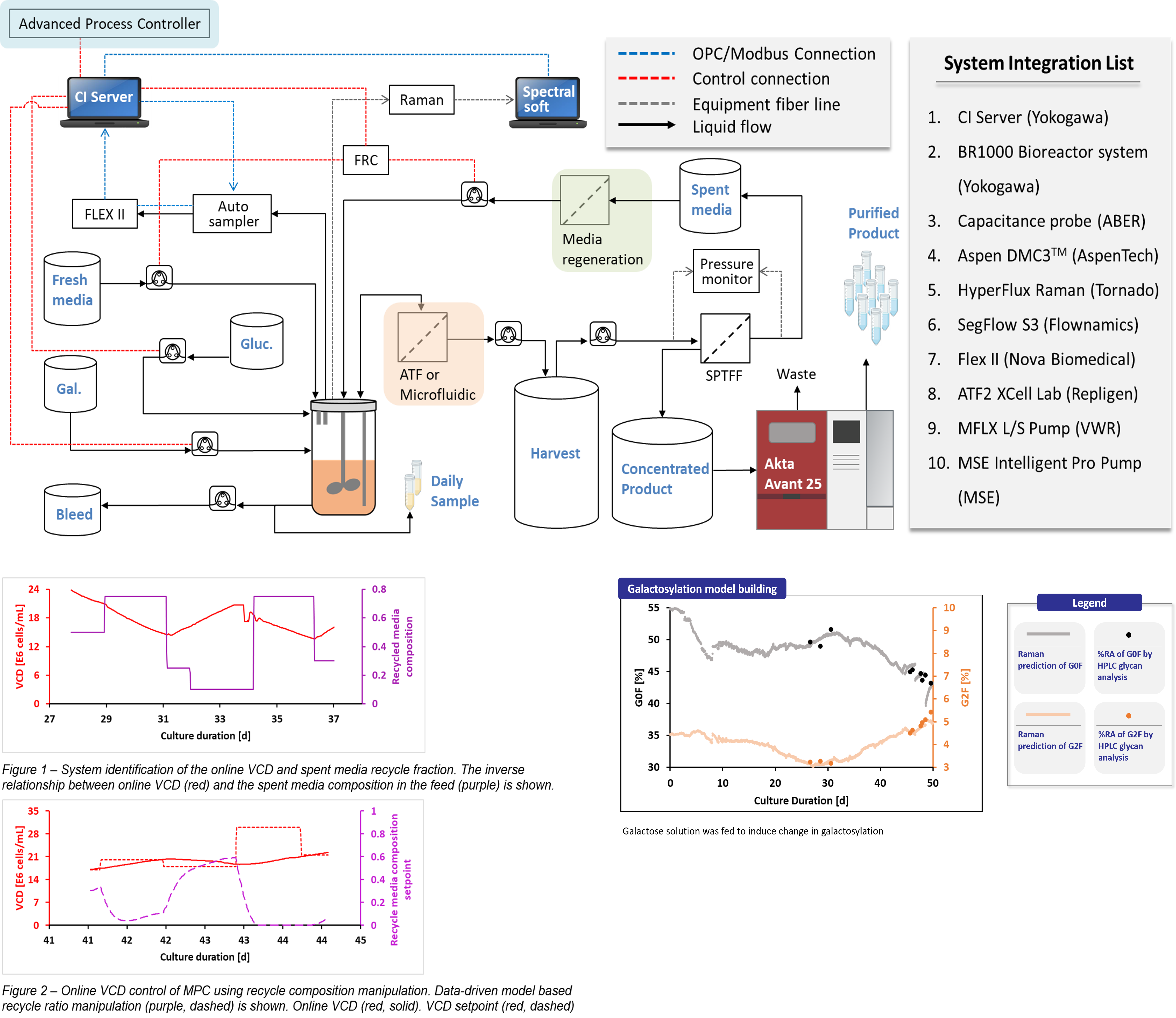
To address these limitations, we propose an innovative platform integrating a microfluidic-based perfusion device, a spent medium recycling and regeneration system, and a multi-input multi-output (MIMO) model predictive controller (MPC). Our integrated continuous upstream system effectively recycles spent medium while maintaining cell growth and mAb production. Our studies demonstrate that perfusion cultures require significantly lower nutrient input to sustain cell viability and productivity. Notably, increasing residence time or recycling spent medium can supply sufficient nutrients, as evidenced by minimal impact on cell density, viability, and productivity at up to 75% spent medium recycling in perfusion cultures using NISTCHO and AMBIC media.
To further enhance process control, we implemented an automated feedback control system for productivity and N-linked glycosylation, utilizing Raman spectroscopy-based soft sensors (Tornado Raman), Aspen’s dynamic matrix control (AspenTech), and the BR1000 bioreactor platform (Yokogawa). Additionally, we developed a novel electrokinetic medium regeneration system to selectively remove growth-inhibiting byproducts, such as lactate and ammonia, while preserving essential nutrients. Preliminary results indicate that regenerated media support higher viable cell densities compared to recycled media without lactate and ammonia removal.
This platform represents a significant advancement in upstream biomanufacturing by improving process efficiency, product consistency, while reducing COGs. Furthermore, its adaptability extends to cell and gene therapy manufacturing, where precise control of product titer and quality attributes is essential for flexible production scales.