2025 AIChE Annual Meeting
(588cp) Insights into Molybdenum Impregnation on TiO? Via 2D Correlation Raman Spectroscopy
Specific to the manufacturing of TiO2, the sulfate process is a common way to treat ilmenite. During the process, sulfuric acid is used to extract iron(II) sulfate pentahydrate.[1] This process will retain some sulfate residue in TiO2 pellets. This research also shows how sulfate residues affect the adsorption of metal ions during the impregnation.
To Probe these effects, in-situ Raman spectroscopy is employed during the impregnation step to track changes in precursor concentration and derive impregnation kinetics. Additionally, 2D correlation spectroscopy is applied to the collected spectroscopic data, reducing spectral perturbations and revealing the true interaction dynamics. This technique enhances data resolution and clarity, offering precise insights into how impregnated materials interact with various adsorbates under different conditions, such as variations in pH and the presence of monomers or oligomers.
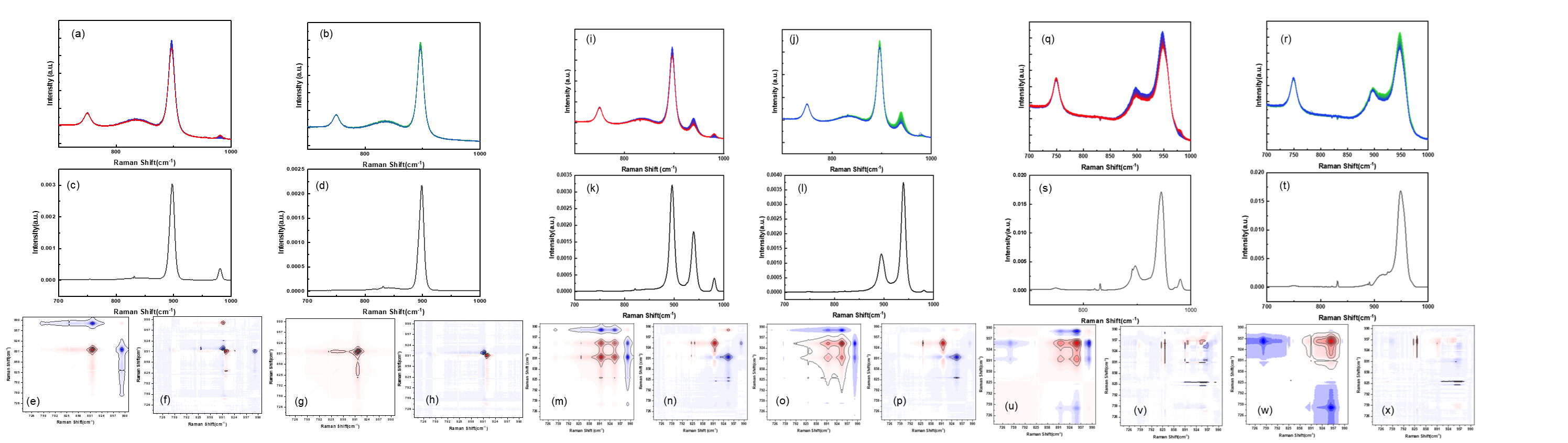
According to the literature, the structure of molybdenum oxide species deposited on a support surface is governed by the pH value of the precursor solution.[2] At pH 9, only molybdenum oxide monomer (MoO42-) is present, giving rise to a characteristic Raman peak at 896 cm-1, which corresponds to the breathing mode of the (MoO4)2- ion. At pH 6 and 5, both monomers (MoO42-) and oligomers (Mo7O246-) coexist in the solution, indicated by peaks at 896 cm-1 and the 939 cm-1, respectively. The intensities of these peaks reflect the changing concentrations of molybdenum species in solution due to their deposition/adsorption on the TiO2 surface. After spectral normalization we observe a clear decay in the concentration of both monomers and oligomers. In the case of untreated support pellets, this process is accompanied by the gradual emergence of a peak at 980 cm⁻¹, corresponding to the leaching of residual sulfate (SO₄²⁻) species from the support surface. In contrast, pellets pre-treated with NH₄OH to remove sulfate show no such sulfate peak. Notably, while the adsorption behavior of monomeric molybdenum species remains largely unaffected by the sulfate treatment, a significant change is observed in both the extent and rate of oligomer adsorption. By fitting adsorption isotherm models with both pseudo-first order and second order behavior, we find that untreated supports can anchor a larger amount of molybdenum oxide species per unit of surface area.
Two-dimensional correlation spectroscopy (2D-COS) is a mathematical approach used to analyze variations in spectral signals under the influence of an external perturbation.[3,4] In this study, the measured signals are Raman shifts corresponding to molybdenum oxide species and sulfate groups, with time serving as the perturbation. 2D-COS is typically divided into synchronous and asynchronous spectra. The synchronous spectrum captures simultaneous or correlated intensity changes between two spectral features. Peaks that appear off the diagonal represent correlations between different spectral bands, while peaks along the diagonal—known as auto-peaks—reflect self-correlated variations in individual bands. These auto-peaks can also be represented as auto-power spectra, which are always positive and quantify the overall extent of Raman intensity variation during the impregnation process. In contrast, the asynchronous spectrum reveals sequential or out-of-phase changes in spectral intensity. Asynchronous cross-peaks emerge only when two spectral features vary independently over time, providing insights into the relative timing of molecular interactions or structural transitions.
Figure (e) to figure (h) represent the 2D-COS analysis of impregnation for both treated and untreated TiO2 pellets at pH 9. The auto-power spectra reveal Raman shift at 896 cm-1 in both samples, and at 980 cm-1 in the untreated pellets. In this case, there is no significant difference in adsorption between treated and intreated pellets. The negative cross-peak at Ф (896,980) indicates an increase in intensity of the peak 980 cm-1 due to sulfate species leaching and the decreasing of the intensity of the peak 896 cm-1 due to the adsorption of the molybdenum monomer. The presence of a positive cross-peak at Ψ(896, 980) alongside the negative Φ(896, 980) suggests that the leaching of sulfate species precedes the adsorption of the molybdenum monomer. This sequential relationship highlights the dynamic interplay between surface-bound sulfate groups and molybdenum precursor species during the early stages of impregnation.
Figure (m) to figure (p) shows the 2D-COS of impregnation process for both treated and untreated TiO2 at pH 6. The auto-power spectra reveal changes in the Raman shift at 896 cm-1, 939 cm-1, and 980 cm-1, which correspond to variations in the concentration of molybdenum monomer (MoO₄²⁻), molybdenum polymer (Mo₇O₂₄⁶⁻) and sulfate (SO₄²⁻) groups, respectively. In the synchronous spectrum, the positive cross-peak at Φ(896, 939) indicates that the concentrations of molybdenum monomer and oligomer change in the same direction—either increasing or decreasing together during impregnation. Conversely, negative cross-peaks at Φ(896, 980) and Φ(939, 980) suggest that the adsorption of molybdenum species is accompanied by an increase in the sulfate signal, consistent with sulfate leaching from the TiO₂ surface. The reciprocal cross-peaks—Φ(939, 896), Φ(980, 896), and Φ(980, 939)—show symmetric relationships and confirm these interactions. To determine the sequence of molecular events, both synchronous and asynchronous spectra are analyzed together. The cross-peak (896, 939) is positive in both spectra, indicating that the adsorption of the molybdenum monomer precedes that of the oligomer. The cross-peak (939, 980) is negative in the synchronous spectrum and positive in the asynchronous spectrum, suggesting that the leaching of sulfate occurs before the adsorption of the molybdenum oligomer. The overall adsorption sequence is thus:
Step 1: Leaching of the sulfate group from the TiO2 pellets.
Step 2: Adsorption of molybdenum monomer by the TiO2 pellets.
Step 3: Adsorption of the molybdenum oligomer by the TiO2 pellets.
At pH 5, the ratio of oligomer to monomer increases, with oligomeric species becoming more dominant. This shift results in greater adsorption of molybdenum oligomers compared to monomers, as confirmed by the auto-power spectra, which display a stronger signal at 939 cm⁻¹ than at 896 cm⁻¹ under these conditions.
This study demonstrates that the adsorption of molybdenum oxide species onto TiO₂ supports is strongly influenced by both pH and residual sulfate content. In-situ Raman spectroscopy reveals that monomeric MoO₄²⁻ dominates at pH 9, while oligomeric Mo₇O₂₄⁶⁻ becomes more prevalent at lower pH values. Residual sulfate, retained from TiO₂ manufacturing, promotes the adsorption of oligomers without significantly affecting monomer adsorption, resulting in higher molybdenum loading on untreated supports. Two-dimensional correlation spectroscopy further reveals the sequential nature of these interactions, showing that sulfate leaching occurs prior to monomer adsorption, which is followed by oligomer deposition. These findings provide valuable insights for optimizing catalyst impregnation processes, allowing for precise control over metal oxide dispersion and loading. The combined application of in-situ spectroscopy and advanced spectral analysis offers a powerful method for investigating dynamic interfacial phenomena during catalyst synthesis.
Reference:
- Sadeghi, M. H.; Nasr Esfahany, M., Development of a Safe and Environmentally Friendly Sulfate Process for the Production of Titanium Oxide. Industrial & Engineering Chemistry Research 2022, 61 (4), 1786-1796.
- Zhang, S.; Han, M., Effect of synthesis pH on the structure and catalytic properties of FeMo catalysts. RSC Advances 2019, 9 (71), 41720-41728.
- I. Noda, J. Am. Chem. Soc. 111 (1989) 8116–8118.
- I. Noda, Y. Ozaki, Two-Dimensional Correlation Spectroscopy — Applications in Vibrational and Optical Spectroscopy, John Wiley & Sons Ltd., Chinchester, U.K, 2004.